Testhyperlink123: Difference between revisions
Jump to navigation
Jump to search
Guptareeya53 (talk | contribs) No edit summary |
Guptareeya53 (talk | contribs) No edit summary |
||
| Line 36: | Line 36: | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G32254QI G32254QI] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G32254QI G32254QI] <br> | ||
'''term_in_sentence''' : Thus, malignancy of MCF-7 is highly dependent on monosialyl-Gb5, and its activation of cSrc and FAK in GEM.[PMID:[https://pubmed.ncbi.nlm.nih.gov/12401210 12401210]] <br> | '''term_in_sentence''' : Thus, malignancy of MCF-7 is highly dependent on monosialyl-Gb5, and its activation of cSrc and FAK in GEM.[PMID:[https://pubmed.ncbi.nlm.nih.gov/12401210 12401210]] <br> | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/12401210 12401210]|[https://pubmed.ncbi.nlm.nih.gov/16995838 16995838]| | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/12401210 12401210]|[https://pubmed.ncbi.nlm.nih.gov/16995838 16995838]|[https://pubmed.ncbi.nlm.nih.gov/27318475 27318475] <br> | ||
'''definition''' : alpha-Neup5Ac-(2->3)-beta-D-Galp-(1->3)-beta-D-GalpNAc-(1->3)-alpha-D-Galp-(1->4)-beta-D-Galp-(1->4)-beta-D-Glcp is a linear amino hexasaccharide comprising β-D-glucose at the reducing end with at the 4-position a β-D-galactosyl-(1→3)-N-acetyl-β-D-glucosaminyl-(1→3)-α-D-galactosyl-(1→4)-β-D-galactosyl moiety, α(2→3)-sialylated at the terminal galactosyl residue.[CHEBI:62313] <br> | '''definition''' : alpha-Neup5Ac-(2->3)-beta-D-Galp-(1->3)-beta-D-GalpNAc-(1->3)-alpha-D-Galp-(1->4)-beta-D-Galp-(1->4)-beta-D-Glcp is a linear amino hexasaccharide comprising β-D-glucose at the reducing end with at the 4-position a β-D-galactosyl-(1→3)-N-acetyl-β-D-glucosaminyl-(1→3)-α-D-galactosyl-(1→4)-β-D-galactosyl moiety, α(2→3)-sialylated at the terminal galactosyl residue.[CHEBI:62313] <br> | ||
'''term_xref''' : GTC:G32254QI|GlycoEpitope:EP0099|GlycoMotif:GGM.000080|CID: | '''term_xref''' : GTC:G32254QI|GlycoEpitope:EP0099|GlycoMotif:GGM.000080|CID:73427363|CHEBI:62313 <br> | ||
'''synonyms''' : GL-7 globoseries ganglioside (SSEA-4)|Monosialyl Gb5|Sialosyl galactosyl globoside|GL-7 globoseries ganglioside|SSEA-4 <br> | '''synonyms''' : GL-7 globoseries ganglioside (SSEA-4)|Monosialyl Gb5|Sialosyl galactosyl globoside|GL-7 globoseries ganglioside|SSEA-4 <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
| Line 82: | Line 82: | ||
'''glycan_dictionary_accession''' : GSD000128 <br> | '''glycan_dictionary_accession''' : GSD000128 <br> | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G79389NT G79389NT] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G79389NT G79389NT] <br> | ||
'''term_in_sentence''' : The appearance of the N-acetyl GM2 antigen correlated well with the degree of differentiation of the cancer cells in patients with squamous cell carcinoma and adenocarcinoma of the lung.[PMID: | '''term_in_sentence''' : The appearance of the N-acetyl GM2 antigen correlated well with the degree of differentiation of the cancer cells in patients with squamous cell carcinoma and adenocarcinoma of the lung.[PMID:[https://pubmed.ncbi.nlm.nih.gov/3167861 3167861]] <br> | ||
'''publication''' : | '''publication''' :[https://pubmed.ncbi.nlm.nih.gov/3167861 3167861]|[https://pubmed.ncbi.nlm.nih.gov/2153431 2153431]| [https://pubmed.ncbi.nlm.nih.gov/25673107 25673107] <br> | ||
'''definition''' : beta-D-GalpNAc-(1->4)-[alpha-Neup5Ac-(2->3)]-beta-D-Galp-(1->4)-beta-D-Glcp is a branched amino tetrasaccharide consisting of the linear sequence β-D-GalNAc-(1→4)-β-D-Gal-(1→4)-β-D-Glc having a Neu5Ac residue attached to the galactose via an α-(2→3) linkage. Corresponds to the carbohydrate portion of ganglioside GM2.[CHEBI:59220] <br> | '''definition''' : beta-D-GalpNAc-(1->4)-[alpha-Neup5Ac-(2->3)]-beta-D-Galp-(1->4)-beta-D-Glcp is a branched amino tetrasaccharide consisting of the linear sequence β-D-GalNAc-(1→4)-β-D-Gal-(1→4)-β-D-Glc having a Neu5Ac residue attached to the galactose via an α-(2→3) linkage. Corresponds to the carbohydrate portion of ganglioside GM2.[CHEBI:59220] <br> | ||
'''term_xref''' : GTC:G79389NT|GlycoEpitope:EP0051|CID: | '''term_xref''' : GTC:G79389NT|GlycoEpitope:EP0051|CID: 45266845|CHEBI:59220 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : The biological role played by a material entity when bound by a receptor of the adaptive immune system. Specific site on an antigen to which an antibody binds.[CHEBI:59220] <br> | '''function''' : The biological role played by a material entity when bound by a receptor of the adaptive immune system. Specific site on an antigen to which an antibody binds.[CHEBI:59220] <br> | ||
| Line 99: | Line 99: | ||
'''glytoucan_accession ''' : [https://glygen.org/glycan/G00056MO G00056MO]<br> | '''glytoucan_accession ''' : [https://glygen.org/glycan/G00056MO G00056MO]<br> | ||
'''term_in_sentence''' : The N-acetyllactosamine type 1 (LacNAc type 1, Galβ1,3GlcNAc) is a well-known precursor of several important blood group epitopes, such as Lewis A, Lewis B, or sialyl Lewis A [1], which are involved in many biological processes, e.g., fertilization [2] and pathogen adhesion [3]. [PMID:[https://pubmed.ncbi.nlm.nih.gov/28796164 28796164]]<br> | '''term_in_sentence''' : The N-acetyllactosamine type 1 (LacNAc type 1, Galβ1,3GlcNAc) is a well-known precursor of several important blood group epitopes, such as Lewis A, Lewis B, or sialyl Lewis A [1], which are involved in many biological processes, e.g., fertilization [2] and pathogen adhesion [3]. [PMID:[https://pubmed.ncbi.nlm.nih.gov/28796164 28796164]]<br> | ||
'''publication''' : | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/28796164 28796164]|[https://pubmed.ncbi.nlm.nih.gov/28167527 28167527]<br> | ||
'''definition''' : <br> | '''definition''' : <br> | ||
'''term_xref''' : GTC:G00056MO|GlycoMotif:GGM.000005<br> | '''term_xref''' : GTC:G00056MO|GlycoMotif:GGM.000005<br> | ||
| Line 130: | Line 130: | ||
'''glycan_dictionary_accession''' : GSD000130 <br> | '''glycan_dictionary_accession''' : GSD000130 <br> | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G56478LD G56478LD] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G56478LD G56478LD] <br> | ||
'''term_in_sentence''' : The monoclonal antibodies MK2-34 and MK1-16 (both IgM), which specifically detect N-glycolyl GM2 and N-acetyl GM2, respectively, were generated by immunizing mice with liposomes containing monophosphoryl lipid A, trehalose dimycolate, and the antigenic ganglioside.[PMID: | '''term_in_sentence''' : The monoclonal antibodies MK2-34 and MK1-16 (both IgM), which specifically detect N-glycolyl GM2 and N-acetyl GM2, respectively, were generated by immunizing mice with liposomes containing monophosphoryl lipid A, trehalose dimycolate, and the antigenic ganglioside.[PMID:[https://pubmed.ncbi.nlm.nih.gov/3167861 3167861]] <br> | ||
'''publication''' : [ | '''publication''' : [[https://pubmed.ncbi.nlm.nih.gov/3167861 3167861]|[https://pubmed.ncbi.nlm.nih.gov/11861662 11861662]|[https://pubmed.ncbi.nlm.nih.gov/17980710 17980710]|[https://pubmed.ncbi.nlm.nih.gov/3731079 3731079]| [https://pubmed.ncbi.nlm.nih.gov/25673107 25673107]] <br> | ||
'''definition''' : (2S,4S,5R,6R)-2-[(2R,3S,4R,5R,6S)-3-[(2S,3R,4R,5R,6R)-3-Acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-5-hydroxy-2-(hydroxymethyl)-6-[(2R,3S,4R,5R,6R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-4-yl]oxy-4-hydroxy-5-[(2-hydroxyacetyl)amino]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid is a member of carbohydrates and carbohydrate derivatives.[CHEBI:151464] <br> | '''definition''' : (2S,4S,5R,6R)-2-[(2R,3S,4R,5R,6S)-3-[(2S,3R,4R,5R,6R)-3-Acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-5-hydroxy-2-(hydroxymethyl)-6-[(2R,3S,4R,5R,6R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-4-yl]oxy-4-hydroxy-5-[(2-hydroxyacetyl)amino]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid is a member of carbohydrates and carbohydrate derivatives.[CHEBI:151464] <br> | ||
'''term_xref''' : GTC:G56478LD |GlycoEpitope:EP0052|CID: | '''term_xref''' : GTC:G56478LD |GlycoEpitope:EP0052|CID:91845742|CHEBI:151464|GlycoEpitope:GGM.000096 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
| Line 147: | Line 147: | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G78059CC G78059CC] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G78059CC G78059CC] <br> | ||
'''term_in_sentence''' : Our study identifies extracellular N-linked glycans-and the glycoprotein neurexin 1β specifically-as key modulators of neuronal uptake of αSacetyl, drawing attention to the potential therapeutic value of αSacetyl-glycan interactions.[PMID:[https://pubmed.ncbi.nlm.nih.gov/31211781 31211781]] <br> | '''term_in_sentence''' : Our study identifies extracellular N-linked glycans-and the glycoprotein neurexin 1β specifically-as key modulators of neuronal uptake of αSacetyl, drawing attention to the potential therapeutic value of αSacetyl-glycan interactions.[PMID:[https://pubmed.ncbi.nlm.nih.gov/31211781 31211781]] <br> | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/31211781 31211781]|[https://pubmed.ncbi.nlm.nih.gov/28384382 28384382]|[https://pubmed.ncbi.nlm.nih.gov/30305477 30305477]|[https://pubmed.ncbi.nlm.nih.gov/31364612 31364612]|[https://pubmed.ncbi.nlm.nih.gov/26899268 26899268]|[https://pubmed.ncbi.nlm.nih.gov/29945790 29945790]|[https://pubmed.ncbi.nlm.nih.gov/29652253 29652253]|[https://pubmed.ncbi.nlm.nih.gov/29723274 29723274]|[https://pubmed.ncbi.nlm.nih.gov/30962676 30962676]|[https://pubmed.ncbi.nlm.nih.gov/29778448 29778448]|[https://pubmed.ncbi.nlm.nih.gov/25325701 25325701]|[https://pubmed.ncbi.nlm.nih.gov/31100702 31100702]|[https://pubmed.ncbi.nlm.nih.gov/28898525 28898525]|[https://pubmed.ncbi.nlm.nih.gov/22944671 22944671]|[https://pubmed.ncbi.nlm.nih.gov/19277537 19277537]|[https://pubmed.ncbi.nlm.nih.gov/24473128 24473128]|[https://pubmed.ncbi.nlm.nih.gov/17957771 17957771]|[https://pubmed.ncbi.nlm.nih.gov/27554083 27554083]|[https://pubmed.ncbi.nlm.nih.gov/15189166 15189166]|[https://pubmed.ncbi.nlm.nih.gov/11269317 11269317]| | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/31211781 31211781]|[https://pubmed.ncbi.nlm.nih.gov/28384382 28384382]|[https://pubmed.ncbi.nlm.nih.gov/30305477 30305477]|[https://pubmed.ncbi.nlm.nih.gov/31364612 31364612]|[https://pubmed.ncbi.nlm.nih.gov/26899268 26899268]|[https://pubmed.ncbi.nlm.nih.gov/29945790 29945790]|[https://pubmed.ncbi.nlm.nih.gov/29652253 29652253]|[https://pubmed.ncbi.nlm.nih.gov/29723274 29723274]|[https://pubmed.ncbi.nlm.nih.gov/30962676 30962676]|[https://pubmed.ncbi.nlm.nih.gov/29778448 29778448]|[https://pubmed.ncbi.nlm.nih.gov/25325701 25325701]|[https://pubmed.ncbi.nlm.nih.gov/31100702 31100702]|[https://pubmed.ncbi.nlm.nih.gov/28898525 28898525]|[https://pubmed.ncbi.nlm.nih.gov/22944671 22944671]|[https://pubmed.ncbi.nlm.nih.gov/19277537 19277537]|[https://pubmed.ncbi.nlm.nih.gov/24473128 24473128]|[https://pubmed.ncbi.nlm.nih.gov/17957771 17957771]|[https://pubmed.ncbi.nlm.nih.gov/27554083 27554083]|[https://pubmed.ncbi.nlm.nih.gov/15189166 15189166]|[https://pubmed.ncbi.nlm.nih.gov/11269317 11269317]|[https://pubmed.ncbi.nlm.nih.gov/19577919 19577919]|[https://pubmed.ncbi.nlm.nih.gov/24303005 24303005]|[https://pubmed.ncbi.nlm.nih.gov/12042249 12042249]|[https://pubmed.ncbi.nlm.nih.gov/22191536 22191536]|[https://pubmed.ncbi.nlm.nih.gov/28324664 28324664]|[https://pubmed.ncbi.nlm.nih.gov/25965797 25965797]|[https://pubmed.ncbi.nlm.nih.gov/25434632 25434632]|[https://pubmed.ncbi.nlm.nih.gov/30484244 30484244]|[https://pubmed.ncbi.nlm.nih.gov/28110657 28110657]| [https://pubmed.ncbi.nlm.nih.gov/29865036 29865036]|[https://pubmed.ncbi.nlm.nih.gov/20129637 20129637]|[https://pubmed.ncbi.nlm.nih.gov/27346875 27346875]|[https://pubmed.ncbi.nlm.nih.gov/30280442 30280442]|[https://pubmed.ncbi.nlm.nih.gov/26911286 26911286]|[https://pubmed.ncbi.nlm.nih.gov/22491358 22491358]|[https://pubmed.ncbi.nlm.nih.gov/26582281 26582281]|[https://pubmed.ncbi.nlm.nih.gov/26398792 26398792]|[https://pubmed.ncbi.nlm.nih.gov/31548313 31548313]|[https://pubmed.ncbi.nlm.nih.gov/31066275 31066275]|[https://pubmed.ncbi.nlm.nih.gov/28331984 28331984]|[https://pubmed.ncbi.nlm.nih.gov/25482090 25482090]| [https://pubmed.ncbi.nlm.nih.gov/28518173 28518173]|[https://pubmed.ncbi.nlm.nih.gov/30733536 30733536]|[https://pubmed.ncbi.nlm.nih.gov/22326428 22326428]|[https://pubmed.ncbi.nlm.nih.gov/30063825 30063825]|[https://pubmed.ncbi.nlm.nih.gov/30315103 30315103]|[https://pubmed.ncbi.nlm.nih.gov/21558494 21558494]|[https://pubmed.ncbi.nlm.nih.gov/31676555 31676555]|[https://pubmed.ncbi.nlm.nih.gov/31256374 31256374] <br> | ||
'''definition''' : The carbohydrate portion of a glycoprotein that has a glycan linked through the nitrogen of an asparagine side-chain.[CHEBI:59520] <br> | '''definition''' : The carbohydrate portion of a glycoprotein that has a glycan linked through the nitrogen of an asparagine side-chain.[CHEBI:59520] <br> | ||
'''term_xref''' : GTC:G78059CC|CID: | '''term_xref''' : GTC:G78059CC|CID: 70679232|CHEBI:70967 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : N-glycans modulate the function of several cell surface proteins which involved in migration, adhesion and which are responsible for regulating myelination.[PMID: 25151374]|Affect transportation of the glycosylated proteins in the Golgi.[PMID: 25151374]|Affects cell-cell interaction, adhesion, expression, proper folding.[PMID: 25151374]|extracellular N-glycans act as key modulators of neuronal uptake of αacetyl [PMID: [https://pubmed.ncbi.nlm.nih.gov/31211781 31211781]]. <br> | '''function''' : N-glycans modulate the function of several cell surface proteins which involved in migration, adhesion and which are responsible for regulating myelination.[PMID: 25151374]|Affect transportation of the glycosylated proteins in the Golgi.[PMID: 25151374]|Affects cell-cell interaction, adhesion, expression, proper folding.[PMID: 25151374]|extracellular N-glycans act as key modulators of neuronal uptake of αacetyl [PMID: [https://pubmed.ncbi.nlm.nih.gov/31211781 31211781]]. <br> | ||
| Line 161: | Line 161: | ||
'''glycan_dictionary_accession''' : GSD000132 <br> | '''glycan_dictionary_accession''' : GSD000132 <br> | ||
'''glytoucan_accession ''' : <br> | '''glytoucan_accession ''' : <br> | ||
'''term_in_sentence''' : In the carbohydrate moiety , alpha-subunit from normal pregnancy hCG contained nonfucosylated , mono- and biantennary N-linked structures (49.3 and 36.7%, means); fucosylated biantennary and triantennary oligosaccharides were also identified (7.3 and 6.9%).[PMID: | '''term_in_sentence''' : In the carbohydrate moiety , alpha-subunit from normal pregnancy hCG contained nonfucosylated , mono- and biantennary N-linked structures (49.3 and 36.7%, means); fucosylated biantennary and triantennary oligosaccharides were also identified (7.3 and 6.9%).[PMID:[https://pubmed.ncbi.nlm.nih.gov/9449027 9449027]] <br> | ||
'''publication''' : | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/9449027 9449027] |[https://pubmed.ncbi.nlm.nih.gov/30258536 30258536]|[https://pubmed.ncbi.nlm.nih.gov/23554380 23554380]|[https://pubmed.ncbi.nlm.nih.gov/16675584 16675584]|[https://pubmed.ncbi.nlm.nih.gov/26747427 26747427]|[https://pubmed.ncbi.nlm.nih.gov/22457527 22457527]|[https://pubmed.ncbi.nlm.nih.gov/24362443 24362443]|[https://pubmed.ncbi.nlm.nih.gov/19657637 19657637]|[https://pubmed.ncbi.nlm.nih.gov/8811881 8811881]|[https://pubmed.ncbi.nlm.nih.gov/19218011 19218011]|[https://pubmed.ncbi.nlm.nih.gov/20022111 20022111]|[https://pubmed.ncbi.nlm.nih.gov/17363544 17363544]|[https://pubmed.ncbi.nlm.nih.gov/17368483 17368483]|[https://pubmed.ncbi.nlm.nih.gov/20564614 20564614]|[https://pubmed.ncbi.nlm.nih.gov/27054024 27054024]|[https://pubmed.ncbi.nlm.nih.gov/28397880 28397880]|[https://pubmed.ncbi.nlm.nih.gov/31490688 31490688]|[https://pubmed.ncbi.nlm.nih.gov/31860772 31860772]|[https://pubmed.ncbi.nlm.nih.gov/17012310 17012310]|[https://pubmed.ncbi.nlm.nih.gov/29633346 29633346]|[https://pubmed.ncbi.nlm.nih.gov/9442021 9442021]|[https://pubmed.ncbi.nlm.nih.gov/30350565 30350565]|[https://pubmed.ncbi.nlm.nih.gov/30296068 30296068]|[https://pubmed.ncbi.nlm.nih.gov/31800250 31800250]|[https://pubmed.ncbi.nlm.nih.gov/30021910 30021910]|[https://pubmed.ncbi.nlm.nih.gov/31133022 31133022]| [https://pubmed.ncbi.nlm.nih.gov/29224385 29224385]|[https://pubmed.ncbi.nlm.nih.gov/[https://pubmed.ncbi.nlm.nih.gov/28630087 28630087] [https://pubmed.ncbi.nlm.nih.gov/28630087 28630087]]|[https://pubmed.ncbi.nlm.nih.gov/26444434 26444434]|[https://pubmed.ncbi.nlm.nih.gov/31697975 31697975]|[https://pubmed.ncbi.nlm.nih.gov/30991260 30991260] <br> | ||
'''definition''' : glycans without a fucose component/monosaccharide residue. <br> | '''definition''' : glycans without a fucose component/monosaccharide residue. <br> | ||
'''term_xref''' : <br> | '''term_xref''' : <br> | ||
| Line 176: | Line 176: | ||
'''glycan_dictionary_accession''' : GSD000133 <br> | '''glycan_dictionary_accession''' : GSD000133 <br> | ||
'''glytoucan_accession ''' : <br> | '''glytoucan_accession ''' : <br> | ||
'''term_in_sentence''' : Structural analysis combining methylation-mass spectrometry and 400 MHz 1H-n.m.r. spectrometry of oligosaccharide alditols released from human leucocyte lactotransferrin shows the presence of two disialylated and non-fucosylated biantennary glycans of the N-acetyl-lactosaminic type.[PMID: | '''term_in_sentence''' : Structural analysis combining methylation-mass spectrometry and 400 MHz 1H-n.m.r. spectrometry of oligosaccharide alditols released from human leucocyte lactotransferrin shows the presence of two disialylated and non-fucosylated biantennary glycans of the N-acetyl-lactosaminic type.[PMID: [https://pubmed.ncbi.nlm.nih.gov/2390069 2390069]] <br> | ||
'''publication''' : | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/2390069 2390069]| [https://pubmed.ncbi.nlm.nih.gov/9449027 9449027] |[https://pubmed.ncbi.nlm.nih.gov/1375510 1375510]|[https://pubmed.ncbi.nlm.nih.gov/9101715 9101715]|[https://pubmed.ncbi.nlm.nih.gov/2332109 2332109] <br> | ||
'''definition''' : A biantennary glycan with two GlcNAc branches linked to the core and no fucose appears. <br> | '''definition''' : A biantennary glycan with two GlcNAc branches linked to the core and no fucose appears. <br> | ||
'''term_xref''' : <br> | '''term_xref''' : <br> | ||
| Line 191: | Line 191: | ||
'''glycan_dictionary_accession''' : GSD000134 <br> | '''glycan_dictionary_accession''' : GSD000134 <br> | ||
'''glytoucan_accession ''' : <br> | '''glytoucan_accession ''' : <br> | ||
'''term_in_sentence''' : The beta-subunit from normal pregnancy hCG contained fucosylated and nonfucosylated biantennary N-linked structures; however, mono- and triantennary oligosaccharides were also identified (4.6 and 13.7%).[PMID: | '''term_in_sentence''' : The beta-subunit from normal pregnancy hCG contained fucosylated and nonfucosylated biantennary N-linked structures; however, mono- and triantennary oligosaccharides were also identified (4.6 and 13.7%).[PMID:[https://pubmed.ncbi.nlm.nih.gov/9449027 9449027]] <br> | ||
'''publication''' : | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/9449027 9449027] <br> | ||
'''definition''' : A biantennary N-linked glycan with two GlcNAc branches linked to the core and no fucose appears. <br> | '''definition''' : A biantennary N-linked glycan with two GlcNAc branches linked to the core and no fucose appears. <br> | ||
'''term_xref''' : <br> | '''term_xref''' : <br> | ||
| Line 222: | Line 222: | ||
'''glytoucan_accession ''' : <br> | '''glytoucan_accession ''' : <br> | ||
'''term_in_sentence''' : The pattern of nonsialylated oligosaccharides was used for interpretation of the fully sialylated species using bioinformatics tools. From pooled human plasma, we find 90, 101, and 64 different glycan compositions for genetic variants ORM1*F1, ORM1*S, and ORM2, respectively. Glycan structures carry dominantly between 15 and 16 sialic acids indicating an almost complete termination of all antenae with sialic acid.[PMID:[https://pubmed.ncbi.nlm.nih.gov/30295034 30295034]] <br> | '''term_in_sentence''' : The pattern of nonsialylated oligosaccharides was used for interpretation of the fully sialylated species using bioinformatics tools. From pooled human plasma, we find 90, 101, and 64 different glycan compositions for genetic variants ORM1*F1, ORM1*S, and ORM2, respectively. Glycan structures carry dominantly between 15 and 16 sialic acids indicating an almost complete termination of all antenae with sialic acid.[PMID:[https://pubmed.ncbi.nlm.nih.gov/30295034 30295034]] <br> | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/14533820 14533820]|[https://pubmed.ncbi.nlm.nih.gov/1377689 1377689]|[https://pubmed.ncbi.nlm.nih.gov/9705949 9705949]|[https://pubmed.ncbi.nlm.nih.gov/30788437 30788437]|[https://pubmed.ncbi.nlm.nih.gov/28090561 28090561]|[https://pubmed.ncbi.nlm.nih.gov/12498371 12498371]|[https://pubmed.ncbi.nlm.nih.gov/26839900 26839900]| | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/14533820 14533820]|[https://pubmed.ncbi.nlm.nih.gov/1377689 1377689]|[https://pubmed.ncbi.nlm.nih.gov/9705949 9705949]|[https://pubmed.ncbi.nlm.nih.gov/30788437 30788437]|[https://pubmed.ncbi.nlm.nih.gov/28090561 28090561]|[https://pubmed.ncbi.nlm.nih.gov/12498371 12498371]|[https://pubmed.ncbi.nlm.nih.gov/26839900 26839900]| [https://pubmed.ncbi.nlm.nih.gov/31490688 31490688]|[https://pubmed.ncbi.nlm.nih.gov/2656426 2656426]|[https://pubmed.ncbi.nlm.nih.gov/30295034 30295034]|[https://pubmed.ncbi.nlm.nih.gov/31545048 31545048]|[https://pubmed.ncbi.nlm.nih.gov/12381155 12381155]|[https://pubmed.ncbi.nlm.nih.gov/25730103 25730103]|[https://pubmed.ncbi.nlm.nih.gov/26787879 26787879]|[https://pubmed.ncbi.nlm.nih.gov/25646460 25646460]| [https://pubmed.ncbi.nlm.nih.gov/29224385 29224385]|[https://pubmed.ncbi.nlm.nih.gov/26063435 26063435]|[https://pubmed.ncbi.nlm.nih.gov/26954852 26954852]|[https://pubmed.ncbi.nlm.nih.gov/32889432 32889432]|[https://pubmed.ncbi.nlm.nih.gov/2825412 2825412]|[https://pubmed.ncbi.nlm.nih.gov/27504786 27504786]|[https://pubmed.ncbi.nlm.nih.gov/22431161 22431161] <br> | ||
'''definition''' : A glycan which is not sialylated. <br> | '''definition''' : A glycan which is not sialylated. <br> | ||
'''term_xref''' : <br> | '''term_xref''' : <br> | ||
| Line 238: | Line 238: | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G39294YF G39294YF] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G39294YF G39294YF] <br> | ||
'''term_in_sentence''' : Recently, a number of laboratories have shown that O-fucose glycans on the epidermal growth factor (EGF)-like repeats of the Notch extracellular domain modulate Notch signaling.[PMID:[https://pubmed.ncbi.nlm.nih.gov/12417415 12417415]] <br> | '''term_in_sentence''' : Recently, a number of laboratories have shown that O-fucose glycans on the epidermal growth factor (EGF)-like repeats of the Notch extracellular domain modulate Notch signaling.[PMID:[https://pubmed.ncbi.nlm.nih.gov/12417415 12417415]] <br> | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/19594638 19594638]|[https://pubmed.ncbi.nlm.nih.gov/30690220 30690220]| | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/19594638 19594638]|[https://pubmed.ncbi.nlm.nih.gov/30690220 30690220]| [https://pubmed.ncbi.nlm.nih.gov/18952191 18952191]|[https://pubmed.ncbi.nlm.nih.gov/22415200 22415200]|[https://pubmed.ncbi.nlm.nih.gov/12417415 12417415]|[https://pubmed.ncbi.nlm.nih.gov/16400812 16400812]|[https://pubmed.ncbi.nlm.nih.gov/18948267 18948267]|[https://pubmed.ncbi.nlm.nih.gov/21464368 21464368]|[https://pubmed.ncbi.nlm.nih.gov/20816394 20816394]|[https://pubmed.ncbi.nlm.nih.gov/30207383 30207383]|[https://pubmed.ncbi.nlm.nih.gov/20301232 20301232]|[https://pubmed.ncbi.nlm.nih.gov/27129198 27129198]|[https://pubmed.ncbi.nlm.nih.gov/17132502 17132502]|[https://pubmed.ncbi.nlm.nih.gov/22492969 22492969]|[https://pubmed.ncbi.nlm.nih.gov/30030822 30030822]|[https://pubmed.ncbi.nlm.nih.gov/30214079 30214079]|[https://pubmed.ncbi.nlm.nih.gov/26175457 26175457]|[https://pubmed.ncbi.nlm.nih.gov/25378397 25378397]|[https://pubmed.ncbi.nlm.nih.gov/18272537 18272537]|[https://pubmed.ncbi.nlm.nih.gov/28729422 28729422]|[https://pubmed.ncbi.nlm.nih.gov/11524432 11524432]|[https://pubmed.ncbi.nlm.nih.gov/31722217 31722217]|[https://pubmed.ncbi.nlm.nih.gov/23045360 23045360]|[https://pubmed.ncbi.nlm.nih.gov/24803430 24803430]|[https://pubmed.ncbi.nlm.nih.gov/28876865 28876865]|[https://pubmed.ncbi.nlm.nih.gov/25700513 25700513]|[https://pubmed.ncbi.nlm.nih.gov/32518939 32518939]|[https://pubmed.ncbi.nlm.nih.gov/30523691 30523691]|[https://pubmed.ncbi.nlm.nih.gov/18227520 18227520]|[https://pubmed.ncbi.nlm.nih.gov/20816217 20816217]|[https://pubmed.ncbi.nlm.nih.gov/24909690 24909690]|[https://pubmed.ncbi.nlm.nih.gov/17964136 17964136]|[https://pubmed.ncbi.nlm.nih.gov/12036964 12036964]|[https://pubmed.ncbi.nlm.nih.gov/27268051 27268051]|[https://pubmed.ncbi.nlm.nih.gov/15653671 15653671]|[https://pubmed.ncbi.nlm.nih.gov/19948734 19948734]|[https://pubmed.ncbi.nlm.nih.gov/31590629 31590629]|[https://pubmed.ncbi.nlm.nih.gov/32201074 32201074]|[https://pubmed.ncbi.nlm.nih.gov/15189151 15189151]|[https://pubmed.ncbi.nlm.nih.gov/17263732 17263732]|[https://pubmed.ncbi.nlm.nih.gov/32913123 32913123]|[https://pubmed.ncbi.nlm.nih.gov/12909620 12909620]|[https://pubmed.ncbi.nlm.nih.gov/28785176 28785176]|[https://pubmed.ncbi.nlm.nih.gov/17132500 17132500]|[https://pubmed.ncbi.nlm.nih.gov/22949680 22949680]|[https://pubmed.ncbi.nlm.nih.gov/25053492 25053492]|[https://pubmed.ncbi.nlm.nih.gov/28939751 28939751] <br> | ||
'''definition''' : Form of glycosylation where the protein is modified by a fucose residue and can be extended futher to form a tetrasaccharide in certain cases. alpha-linked o-fucose have a consensus motif of C2X4(s/T)C3 where C2 and C3 are conserved cysteines number 2 and 3 respectively of the EGFR like repeats. [Essentials of Glycobiology:[https://www.ncbi.nlm.nih.gov/books/NBK453017/ Chapter13]] <br> | '''definition''' : Form of glycosylation where the protein is modified by a fucose residue and can be extended futher to form a tetrasaccharide in certain cases. alpha-linked o-fucose have a consensus motif of C2X4(s/T)C3 where C2 and C3 are conserved cysteines number 2 and 3 respectively of the EGFR like repeats. [Essentials of Glycobiology:[https://www.ncbi.nlm.nih.gov/books/NBK453017/ Chapter13]] <br> | ||
'''term_xref''' : GTC:G39294YF|GlycoEpitope:EP0005<br> | '''term_xref''' : GTC:G39294YF|GlycoEpitope:EP0005<br> | ||
| Line 256: | Line 256: | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/30464755 30464755]|[https://pubmed.ncbi.nlm.nih.gov/29049853 29049853]|[https://pubmed.ncbi.nlm.nih.gov/29594839 29594839]|[https://pubmed.ncbi.nlm.nih.gov/31654859 31654859]|[https://pubmed.ncbi.nlm.nih.gov/29404877 29404877]|[https://pubmed.ncbi.nlm.nih.gov/30105004 30105004]|[https://pubmed.ncbi.nlm.nih.gov/30669087 30669087]|[https://pubmed.ncbi.nlm.nih.gov/29790000 29790000]|[https://pubmed.ncbi.nlm.nih.gov/28408483 28408483]|[https://pubmed.ncbi.nlm.nih.gov/26862193 26862193]|[https://pubmed.ncbi.nlm.nih.gov/30523150 30523150]|[https://pubmed.ncbi.nlm.nih.gov/30037904 30037904]|[https://pubmed.ncbi.nlm.nih.gov/28408480 28408480]|[https://pubmed.ncbi.nlm.nih.gov/29352075 29352075]|[https://pubmed.ncbi.nlm.nih.gov/29456783 29456783]|[https://pubmed.ncbi.nlm.nih.gov/29577901 29577901]|[https://pubmed.ncbi.nlm.nih.gov/29223644 29223644]|[https://pubmed.ncbi.nlm.nih.gov/28638491 28638491]|[https://pubmed.ncbi.nlm.nih.gov/30221662 30221662]|[https://pubmed.ncbi.nlm.nih.gov/29904918 29904918]|[https://pubmed.ncbi.nlm.nih.gov/32155042 32155042]|[https://pubmed.ncbi.nlm.nih.gov/30298013 30298013]|[https://pubmed.ncbi.nlm.nih.gov/31630803 31630803]|[https://pubmed.ncbi.nlm.nih.gov/31847126 31847126]|[https://pubmed.ncbi.nlm.nih.gov/29784830 29784830]|[https://pubmed.ncbi.nlm.nih.gov/30181664 30181664]|[https://pubmed.ncbi.nlm.nih.gov/28768194 28768194]|[https://pubmed.ncbi.nlm.nih.gov/30134155 30134155]|[https://pubmed.ncbi.nlm.nih.gov/25336654 25336654]|[https://pubmed.ncbi.nlm.nih.gov/30356792 30356792]|[https://pubmed.ncbi.nlm.nih.gov/30199580 30199580]|[https://pubmed.ncbi.nlm.nih.gov/32329777 32329777]|[https://pubmed.ncbi.nlm.nih.gov/28408494 28408494]|[https://pubmed.ncbi.nlm.nih.gov/29774032 29774032]|[https://pubmed.ncbi.nlm.nih.gov/29044951 29044951]|[https://pubmed.ncbi.nlm.nih.gov/23836420 23836420]|[https://pubmed.ncbi.nlm.nih.gov/20301273 20301273]|[https://pubmed.ncbi.nlm.nih.gov/29772801 29772801]|[https://pubmed.ncbi.nlm.nih.gov/28922739 28922739]|[https://pubmed.ncbi.nlm.nih.gov/30100348 30100348]|[https://pubmed.ncbi.nlm.nih.gov/30793403 30793403]|[https://pubmed.ncbi.nlm.nih.gov/30657688 30657688]|[https://pubmed.ncbi.nlm.nih.gov/31237748 31237748]|[https://pubmed.ncbi.nlm.nih.gov/29756380 29756380]|[https://pubmed.ncbi.nlm.nih.gov/29954943 29954943]|[https://pubmed.ncbi.nlm.nih.gov/30018219 30018219]|[https://pubmed.ncbi.nlm.nih.gov/32207184 32207184]|[https://pubmed.ncbi.nlm.nih.gov/25566193 25566193]|[https://pubmed.ncbi.nlm.nih.gov/30626734 30626734]|[https://pubmed.ncbi.nlm.nih.gov/24759912 24759912]| <br> | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/30464755 30464755]|[https://pubmed.ncbi.nlm.nih.gov/29049853 29049853]|[https://pubmed.ncbi.nlm.nih.gov/29594839 29594839]|[https://pubmed.ncbi.nlm.nih.gov/31654859 31654859]|[https://pubmed.ncbi.nlm.nih.gov/29404877 29404877]|[https://pubmed.ncbi.nlm.nih.gov/30105004 30105004]|[https://pubmed.ncbi.nlm.nih.gov/30669087 30669087]|[https://pubmed.ncbi.nlm.nih.gov/29790000 29790000]|[https://pubmed.ncbi.nlm.nih.gov/28408483 28408483]|[https://pubmed.ncbi.nlm.nih.gov/26862193 26862193]|[https://pubmed.ncbi.nlm.nih.gov/30523150 30523150]|[https://pubmed.ncbi.nlm.nih.gov/30037904 30037904]|[https://pubmed.ncbi.nlm.nih.gov/28408480 28408480]|[https://pubmed.ncbi.nlm.nih.gov/29352075 29352075]|[https://pubmed.ncbi.nlm.nih.gov/29456783 29456783]|[https://pubmed.ncbi.nlm.nih.gov/29577901 29577901]|[https://pubmed.ncbi.nlm.nih.gov/29223644 29223644]|[https://pubmed.ncbi.nlm.nih.gov/28638491 28638491]|[https://pubmed.ncbi.nlm.nih.gov/30221662 30221662]|[https://pubmed.ncbi.nlm.nih.gov/29904918 29904918]|[https://pubmed.ncbi.nlm.nih.gov/32155042 32155042]|[https://pubmed.ncbi.nlm.nih.gov/30298013 30298013]|[https://pubmed.ncbi.nlm.nih.gov/31630803 31630803]|[https://pubmed.ncbi.nlm.nih.gov/31847126 31847126]|[https://pubmed.ncbi.nlm.nih.gov/29784830 29784830]|[https://pubmed.ncbi.nlm.nih.gov/30181664 30181664]|[https://pubmed.ncbi.nlm.nih.gov/28768194 28768194]|[https://pubmed.ncbi.nlm.nih.gov/30134155 30134155]|[https://pubmed.ncbi.nlm.nih.gov/25336654 25336654]|[https://pubmed.ncbi.nlm.nih.gov/30356792 30356792]|[https://pubmed.ncbi.nlm.nih.gov/30199580 30199580]|[https://pubmed.ncbi.nlm.nih.gov/32329777 32329777]|[https://pubmed.ncbi.nlm.nih.gov/28408494 28408494]|[https://pubmed.ncbi.nlm.nih.gov/29774032 29774032]|[https://pubmed.ncbi.nlm.nih.gov/29044951 29044951]|[https://pubmed.ncbi.nlm.nih.gov/23836420 23836420]|[https://pubmed.ncbi.nlm.nih.gov/20301273 20301273]|[https://pubmed.ncbi.nlm.nih.gov/29772801 29772801]|[https://pubmed.ncbi.nlm.nih.gov/28922739 28922739]|[https://pubmed.ncbi.nlm.nih.gov/30100348 30100348]|[https://pubmed.ncbi.nlm.nih.gov/30793403 30793403]|[https://pubmed.ncbi.nlm.nih.gov/30657688 30657688]|[https://pubmed.ncbi.nlm.nih.gov/31237748 31237748]|[https://pubmed.ncbi.nlm.nih.gov/29756380 29756380]|[https://pubmed.ncbi.nlm.nih.gov/29954943 29954943]|[https://pubmed.ncbi.nlm.nih.gov/30018219 30018219]|[https://pubmed.ncbi.nlm.nih.gov/32207184 32207184]|[https://pubmed.ncbi.nlm.nih.gov/25566193 25566193]|[https://pubmed.ncbi.nlm.nih.gov/30626734 30626734]|[https://pubmed.ncbi.nlm.nih.gov/24759912 24759912]| <br> | ||
'''definition''' : Form of glycosylation which exclusively occurs in the mitochondrial, cytoplasmic or nuclear compartments of the cell where the protein is modified by a GlcNAc monosaccharide on the serine or threonine residues and is generally not modified or elongated further to form more complex structures. [Essentials of Glycobiology:[https://www.ncbi.nlm.nih.gov/books/NBK453063/ Chapter19]] <br> | '''definition''' : Form of glycosylation which exclusively occurs in the mitochondrial, cytoplasmic or nuclear compartments of the cell where the protein is modified by a GlcNAc monosaccharide on the serine or threonine residues and is generally not modified or elongated further to form more complex structures. [Essentials of Glycobiology:[https://www.ncbi.nlm.nih.gov/books/NBK453063/ Chapter19]] <br> | ||
'''term_xref''' : GTC:G49108TO|GlycoEpitope:EP0004|CID:24139|HMDB: | '''term_xref''' : GTC:G49108TO|GlycoEpitope:EP0004|CID:24139|HMDB:HMDB0000803 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
| Line 268: | Line 268: | ||
'''glycan_dictionary_accession''' : GSD000139 <br> | '''glycan_dictionary_accession''' : GSD000139 <br> | ||
'''glytoucan_accession ''' : <br> | '''glytoucan_accession ''' : <br> | ||
'''term_in_sentence''' : Among these, six fucosylated N-linked glycansand four O-linked glycans exhibited significantly increased expression levels in GC, while five fucosylated N-linked glycans and ten fucosylated O-linked glycans exhibited significantly decreased expression levels in GC.[PMID: | '''term_in_sentence''' : Among these, six fucosylated N-linked glycansand four O-linked glycans exhibited significantly increased expression levels in GC, while five fucosylated N-linked glycans and ten fucosylated O-linked glycans exhibited significantly decreased expression levels in GC.[PMID:[https://pubmed.ncbi.nlm.nih.gov/29865036 29865036]] <br> | ||
'''publication''' : | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/29865036 29865036]|[https://pubmed.ncbi.nlm.nih.gov/30952424 30952424]|[https://pubmed.ncbi.nlm.nih.gov/31611643 31611643]|[https://pubmed.ncbi.nlm.nih.gov/19577919 19577919]|[https://pubmed.ncbi.nlm.nih.gov/32019882 32019882]|[https://pubmed.ncbi.nlm.nih.gov/19139197 19139197]|[https://pubmed.ncbi.nlm.nih.gov/26184710 26184710]|[https://pubmed.ncbi.nlm.nih.gov/11159917 11159917]|[https://pubmed.ncbi.nlm.nih.gov/21536259 21536259]|[https://pubmed.ncbi.nlm.nih.gov/25186198 25186198]|[https://pubmed.ncbi.nlm.nih.gov/19277556 19277556]|[https://pubmed.ncbi.nlm.nih.gov/22261557 22261557]|[https://pubmed.ncbi.nlm.nih.gov/24115046 24115046]|[https://pubmed.ncbi.nlm.nih.gov/18725413 18725413]|[https://pubmed.ncbi.nlm.nih.gov/15966855 15966855]|[https://pubmed.ncbi.nlm.nih.gov/24256304 24256304]|[https://pubmed.ncbi.nlm.nih.gov/25477510 25477510]|[https://pubmed.ncbi.nlm.nih.gov/10359703 10359703]|[https://pubmed.ncbi.nlm.nih.gov/24406064 24406064]|[https://pubmed.ncbi.nlm.nih.gov/22259135 22259135]|[https://pubmed.ncbi.nlm.nih.gov/29279989 29279989]| [https://pubmed.ncbi.nlm.nih.gov/15966855 15966855]|[https://pubmed.ncbi.nlm.nih.gov/30619255 30619255]|[https://pubmed.ncbi.nlm.nih.gov/9673446 9673446]| [https://pubmed.ncbi.nlm.nih.gov/31676555 31676555]|[https://pubmed.ncbi.nlm.nih.gov/28745859 28745859]|[https://pubmed.ncbi.nlm.nih.gov/28196325 28196325]|[https://pubmed.ncbi.nlm.nih.gov/27975143 27975143]|[https://pubmed.ncbi.nlm.nih.gov/30591584 30591584]|[https://pubmed.ncbi.nlm.nih.gov/[https://pubmed.ncbi.nlm.nih.gov/28518173 28518173] [https://pubmed.ncbi.nlm.nih.gov/28518173 28518173]]|[https://pubmed.ncbi.nlm.nih.gov/17522109 17522109]| [https://pubmed.ncbi.nlm.nih.gov/26328495 26328495]|[https://pubmed.ncbi.nlm.nih.gov/32178593 32178593]|[https://pubmed.ncbi.nlm.nih.gov/27529638 27529638]|[https://pubmed.ncbi.nlm.nih.gov/24663386 24663386]|[https://pubmed.ncbi.nlm.nih.gov/22474328 22474328]|[https://pubmed.ncbi.nlm.nih.gov/28096352 28096352]|[https://pubmed.ncbi.nlm.nih.gov/27496768 27496768]| [https://pubmed.ncbi.nlm.nih.gov/18952191 18952191]|[https://pubmed.ncbi.nlm.nih.gov/11135308 11135308]|[https://pubmed.ncbi.nlm.nih.gov/24917611 24917611]|[https://pubmed.ncbi.nlm.nih.gov/11532966 11532966]|[https://pubmed.ncbi.nlm.nih.gov/22786570 22786570]|[https://pubmed.ncbi.nlm.nih.gov/11891229 11891229]|[https://pubmed.ncbi.nlm.nih.gov/18332077 18332077]|[https://pubmed.ncbi.nlm.nih.gov/26867212 26867212]|[https://pubmed.ncbi.nlm.nih.gov/26925665 26925665]|[https://pubmed.ncbi.nlm.nih.gov/16769205 16769205]|[https://pubmed.ncbi.nlm.nih.gov/23475718 23475718]|[https://pubmed.ncbi.nlm.nih.gov/16005634 16005634]|[https://pubmed.ncbi.nlm.nih.gov/28785176 28785176] <br> | ||
'''definition''' : The carbohydrate portion of a glycoprotein that has a glycan linked through the hydroxyl oxygen of serine, threonine, hydroxylysine or hydroxyproline side-chains.[CHEBI:59521] <br> | '''definition''' : The carbohydrate portion of a glycoprotein that has a glycan linked through the hydroxyl oxygen of serine, threonine, hydroxylysine or hydroxyproline side-chains.[CHEBI:59521] <br> | ||
'''term_xref''' : CHEBI:59521 <br> | '''term_xref''' : CHEBI:59521 <br> | ||
| Line 285: | Line 285: | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G59126YU G59126YU] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G59126YU G59126YU] <br> | ||
'''term_in_sentence''' : Protein O-linked mannose beta-1,4-N-acetylglucosaminyltransferase 2 (POMGNT2) catalyzes the first step toward the functional matriglycan structure on alpha-dystroglycan that is responsible for binding extracellular matrix proteins and certain arenaviruses.[PMID: [https://pubmed.ncbi.nlm.nih.gov/27932460 27932460]] <br> | '''term_in_sentence''' : Protein O-linked mannose beta-1,4-N-acetylglucosaminyltransferase 2 (POMGNT2) catalyzes the first step toward the functional matriglycan structure on alpha-dystroglycan that is responsible for binding extracellular matrix proteins and certain arenaviruses.[PMID: [https://pubmed.ncbi.nlm.nih.gov/27932460 27932460]] <br> | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/27932460 27932460]|[https://pubmed.ncbi.nlm.nih.gov/24526361 24526361]|[https://pubmed.ncbi.nlm.nih.gov/25381909 25381909]|[https://pubmed.ncbi.nlm.nih.gov/26644575 26644575]|[https://pubmed.ncbi.nlm.nih.gov/23851827 23851827]|[https://pubmed.ncbi.nlm.nih.gov/28810660 28810660]|[https://pubmed.ncbi.nlm.nih.gov/19054127 19054127]|[https://pubmed.ncbi.nlm.nih.gov/23929950 23929950]|[https://pubmed.ncbi.nlm.nih.gov/21684258 21684258]|[https://pubmed.ncbi.nlm.nih.gov/22608994 22608994]|[https://pubmed.ncbi.nlm.nih.gov/28973932 28973932]|[https://pubmed.ncbi.nlm.nih.gov/24733390 24733390]|[https://pubmed.ncbi.nlm.nih.gov/31851597 31851597]|[https://pubmed.ncbi.nlm.nih.gov/29884773 29884773]|[https://pubmed.ncbi.nlm.nih.gov/28711406 28711406]|[https://pubmed.ncbi.nlm.nih.gov/14577328 14577328]|[https://pubmed.ncbi.nlm.nih.gov/21089343 21089343]|[https://pubmed.ncbi.nlm.nih.gov/14617637 14617637]|[https://pubmed.ncbi.nlm.nih.gov/9838223 9838223]|[https://pubmed.ncbi.nlm.nih.gov/17072003 17072003]| | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/27932460 27932460]|[https://pubmed.ncbi.nlm.nih.gov/24526361 24526361]|[https://pubmed.ncbi.nlm.nih.gov/25381909 25381909]|[https://pubmed.ncbi.nlm.nih.gov/26644575 26644575]|[https://pubmed.ncbi.nlm.nih.gov/23851827 23851827]|[https://pubmed.ncbi.nlm.nih.gov/28810660 28810660]|[https://pubmed.ncbi.nlm.nih.gov/19054127 19054127]|[https://pubmed.ncbi.nlm.nih.gov/23929950 23929950]|[https://pubmed.ncbi.nlm.nih.gov/21684258 21684258]|[https://pubmed.ncbi.nlm.nih.gov/22608994 22608994]|[https://pubmed.ncbi.nlm.nih.gov/28973932 28973932]|[https://pubmed.ncbi.nlm.nih.gov/24733390 24733390]|[https://pubmed.ncbi.nlm.nih.gov/31851597 31851597]|[https://pubmed.ncbi.nlm.nih.gov/29884773 29884773]|[https://pubmed.ncbi.nlm.nih.gov/28711406 28711406]|[https://pubmed.ncbi.nlm.nih.gov/14577328 14577328]|[https://pubmed.ncbi.nlm.nih.gov/21089343 21089343]|[https://pubmed.ncbi.nlm.nih.gov/14617637 14617637]|[https://pubmed.ncbi.nlm.nih.gov/9838223 9838223]|[https://pubmed.ncbi.nlm.nih.gov/17072003 17072003]| [https://pubmed.ncbi.nlm.nih.gov/26328495 26328495]|[https://pubmed.ncbi.nlm.nih.gov/11181560 11181560]|[https://pubmed.ncbi.nlm.nih.gov/31949166 31949166]|[https://pubmed.ncbi.nlm.nih.gov/31988979 31988979]|[https://pubmed.ncbi.nlm.nih.gov/20362685 20362685]|[https://pubmed.ncbi.nlm.nih.gov/27733679 27733679]|[https://pubmed.ncbi.nlm.nih.gov/27493216 27493216]|[https://pubmed.ncbi.nlm.nih.gov/20044576 20044576]|[https://pubmed.ncbi.nlm.nih.gov/30643095 30643095]|[https://pubmed.ncbi.nlm.nih.gov/29732660 29732660]|[https://pubmed.ncbi.nlm.nih.gov/10580142 10580142]|[https://pubmed.ncbi.nlm.nih.gov/14568618 14568618]|[https://pubmed.ncbi.nlm.nih.gov/28079948 28079948]|[https://pubmed.ncbi.nlm.nih.gov/15467391 15467391]|[https://pubmed.ncbi.nlm.nih.gov/27812179 27812179]|[https://pubmed.ncbi.nlm.nih.gov/23761899 23761899]|[https://pubmed.ncbi.nlm.nih.gov/25737452 25737452]|[https://pubmed.ncbi.nlm.nih.gov/32930586 32930586]|[https://pubmed.ncbi.nlm.nih.gov/25381372 25381372]|[https://pubmed.ncbi.nlm.nih.gov/23434682 23434682]|[https://pubmed.ncbi.nlm.nih.gov/16857188 16857188]|[https://pubmed.ncbi.nlm.nih.gov/21452199 21452199]|[https://pubmed.ncbi.nlm.nih.gov/19429925 19429925]|[https://pubmed.ncbi.nlm.nih.gov/25361541 25361541]|[https://pubmed.ncbi.nlm.nih.gov/24297939 24297939]|[https://pubmed.ncbi.nlm.nih.gov/30322079 30322079]|[https://pubmed.ncbi.nlm.nih.gov/23428289 23428289]|[https://pubmed.ncbi.nlm.nih.gov/28427937 28427937]|[https://pubmed.ncbi.nlm.nih.gov/22746206 22746206]|[https://pubmed.ncbi.nlm.nih.gov/27533452 27533452]|[https://pubmed.ncbi.nlm.nih.gov/17502374 17502374]|[https://pubmed.ncbi.nlm.nih.gov/20816174 20816174]|[https://pubmed.ncbi.nlm.nih.gov/27496765 27496765] <br> | ||
'''definition''' : O-linked glycans that are initiated by the addtion of a Man residue to Ser or Thr. Subsequent extension of the Man defines the O-Man core type, either M1, M2, or M3. The M1 type is initated by addition of a GlcNAc in β-linkage to the 2 position of the Man residue. M2 is initiated by addition of another GlcNAc to the 6 position of the M1 core. M3 is initiated by the addition of a GlcNAc in β-linkage to the 4 position of the Man residue. M1 and M2 type core glycans can be extended and branched with Gal, Fuc, Sialic Acid, and GlcA. The M3 core is uniquely extended by a multienzyme system to generate the matriglycan polysaccharide, a protein-specific modification found only on alpha-dystroglycan. [CHEBI:87761] <br> | '''definition''' : O-linked glycans that are initiated by the addtion of a Man residue to Ser or Thr. Subsequent extension of the Man defines the O-Man core type, either M1, M2, or M3. The M1 type is initated by addition of a GlcNAc in β-linkage to the 2 position of the Man residue. M2 is initiated by addition of another GlcNAc to the 6 position of the M1 core. M3 is initiated by the addition of a GlcNAc in β-linkage to the 4 position of the Man residue. M1 and M2 type core glycans can be extended and branched with Gal, Fuc, Sialic Acid, and GlcA. The M3 core is uniquely extended by a multienzyme system to generate the matriglycan polysaccharide, a protein-specific modification found only on alpha-dystroglycan. [CHEBI:87761] <br> | ||
'''term_xref''' : GlycoMotif:GGM.000055|GTC:G59126YU|CHEBI:87761|GlycoEpitope:EP0002|CID: | '''term_xref''' : GlycoMotif:GGM.000055|GTC:G59126YU|CHEBI:87761|GlycoEpitope:EP0002|CID: 91859038<br> | ||
'''synonyms''' : O-mannosyl glycan <br> | '''synonyms''' : O-mannosyl glycan <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
| Line 303: | Line 303: | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/29622537 29622537]|[https://pubmed.ncbi.nlm.nih.gov/26773500 26773500]|[https://pubmed.ncbi.nlm.nih.gov/24046920 24046920]|[https://pubmed.ncbi.nlm.nih.gov/6207232 6207232]|[https://pubmed.ncbi.nlm.nih.gov/8550516 8550516]|[https://pubmed.ncbi.nlm.nih.gov/6609976 6609976]|[https://pubmed.ncbi.nlm.nih.gov/6375319 6375319]|817855|[https://pubmed.ncbi.nlm.nih.gov/2428855 2428855]|[https://pubmed.ncbi.nlm.nih.gov/3312011 3312011]|[https://pubmed.ncbi.nlm.nih.gov/15364312 15364312]|[https://pubmed.ncbi.nlm.nih.gov/1937801 1937801]|[https://pubmed.ncbi.nlm.nih.gov/7729906 7729906] <br> | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/29622537 29622537]|[https://pubmed.ncbi.nlm.nih.gov/26773500 26773500]|[https://pubmed.ncbi.nlm.nih.gov/24046920 24046920]|[https://pubmed.ncbi.nlm.nih.gov/6207232 6207232]|[https://pubmed.ncbi.nlm.nih.gov/8550516 8550516]|[https://pubmed.ncbi.nlm.nih.gov/6609976 6609976]|[https://pubmed.ncbi.nlm.nih.gov/6375319 6375319]|817855|[https://pubmed.ncbi.nlm.nih.gov/2428855 2428855]|[https://pubmed.ncbi.nlm.nih.gov/3312011 3312011]|[https://pubmed.ncbi.nlm.nih.gov/15364312 15364312]|[https://pubmed.ncbi.nlm.nih.gov/1937801 1937801]|[https://pubmed.ncbi.nlm.nih.gov/7729906 7729906] <br> | ||
'''definition''' : An amino pentasaccharide consisting of α-D-galactose, β-D-galactose N-acetyl-α-D-glucosamine, β-D-galactose, and β-D-glucose residues joined in sequence with ((1→4)-, (1→4)-, (1→3)- and (1→4)-linkages, respectively.[CHEBI:68484] <br> | '''definition''' : An amino pentasaccharide consisting of α-D-galactose, β-D-galactose N-acetyl-α-D-glucosamine, β-D-galactose, and β-D-glucose residues joined in sequence with ((1→4)-, (1→4)-, (1→3)- and (1→4)-linkages, respectively.[CHEBI:68484] <br> | ||
'''term_xref''' : GlycoMotif:GGM.000069|GTC:G12460DL|CID: | '''term_xref''' : GlycoMotif:GGM.000069|GTC:G12460DL|CID:71296137|CHEBI:68484|SugarBind_Ligand:160 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
| Line 319: | Line 319: | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/6814203 6814203] <br> | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/6814203 6814203] <br> | ||
'''definition''' : GalNAcβ1→3GalNAcβ1→3Galα1→4Galβ1→4Glcβ1→1Cer [https://doi.org/10.1016/S0040-4039(01)93814-6<nowiki>] </nowiki><br> | '''definition''' : GalNAcβ1→3GalNAcβ1→3Galα1→4Galβ1→4Glcβ1→1Cer [https://doi.org/10.1016/S0040-4039(01)93814-6<nowiki>] </nowiki><br> | ||
'''term_xref''' : GlycoMotif:GGM.000085|GTC:G51699SI|CID: | '''term_xref''' : GlycoMotif:GGM.000085|GTC:G51699SI|CID:56836111|KEGG:G00096|SugarBind_Ligand:19|CHEBI:151565 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
| Line 335: | Line 335: | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/3345524 3345524]|850070|[https://pubmed.ncbi.nlm.nih.gov/10569746 10569746]|[https://pubmed.ncbi.nlm.nih.gov/30624951 30624951]|[https://pubmed.ncbi.nlm.nih.gov/29438961 29438961]|[https://pubmed.ncbi.nlm.nih.gov/6816790 6816790]|[https://pubmed.ncbi.nlm.nih.gov/1551117 1551117]|[https://pubmed.ncbi.nlm.nih.gov/6865961 6865961]|[https://pubmed.ncbi.nlm.nih.gov/25039255 25039255] <br> | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/3345524 3345524]|850070|[https://pubmed.ncbi.nlm.nih.gov/10569746 10569746]|[https://pubmed.ncbi.nlm.nih.gov/30624951 30624951]|[https://pubmed.ncbi.nlm.nih.gov/29438961 29438961]|[https://pubmed.ncbi.nlm.nih.gov/6816790 6816790]|[https://pubmed.ncbi.nlm.nih.gov/1551117 1551117]|[https://pubmed.ncbi.nlm.nih.gov/6865961 6865961]|[https://pubmed.ncbi.nlm.nih.gov/25039255 25039255] <br> | ||
'''definition''' : α-Neup5Ac-(2→3)-β-D-Galp-(1→3)-D-GalpNAc in which the configuration at the GalNAc anomeric carbon is α.[CHEBI:65257] <br> | '''definition''' : α-Neup5Ac-(2→3)-β-D-Galp-(1→3)-D-GalpNAc in which the configuration at the GalNAc anomeric carbon is α.[CHEBI:65257] <br> | ||
'''term_xref''' : GlycoMotif:GGM.000077|GTC:G58896AZ|CID: | '''term_xref''' : GlycoMotif:GGM.000077|GTC:G58896AZ|CID: 21670519|CHEBI:65257|GlycoEpitope:EP0075|SugarBind_Ligand:15 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
| Line 348: | Line 348: | ||
'''glytoucan_accession ''' : <br> | '''glytoucan_accession ''' : <br> | ||
'''term_in_sentence''' : The galactosylation of core fucose (GalFuc epitope) in paucimannose and complex-type N-glycans is characteristic of protostome organisms, including flatworms (planarians).[PMID:[https://pubmed.ncbi.nlm.nih.gov/29475940 29475940]] <br> | '''term_in_sentence''' : The galactosylation of core fucose (GalFuc epitope) in paucimannose and complex-type N-glycans is characteristic of protostome organisms, including flatworms (planarians).[PMID:[https://pubmed.ncbi.nlm.nih.gov/29475940 29475940]] <br> | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/29475940 29475940]|[https://pubmed.ncbi.nlm.nih.gov/29803109 29803109]|[https://pubmed.ncbi.nlm.nih.gov/12603202 12603202]|[https://pubmed.ncbi.nlm.nih.gov/31817246 31817246]|[https://pubmed.ncbi.nlm.nih.gov/31719144 31719144]|[https://pubmed.ncbi.nlm.nih.gov/21057214 21057214]|[https://pubmed.ncbi.nlm.nih.gov/31932305 31932305]|[https://pubmed.ncbi.nlm.nih.gov/17132514 17132514]|[https://pubmed.ncbi.nlm.nih.gov/30382146 30382146]|[https://pubmed.ncbi.nlm.nih.gov/27731363 27731363]|[https://pubmed.ncbi.nlm.nih.gov/31325506 31325506]|[https://pubmed.ncbi.nlm.nih.gov/25746926 25746926]|[https://pubmed.ncbi.nlm.nih.gov/26513758 26513758]|[https://pubmed.ncbi.nlm.nih.gov/16522637 16522637]|[https://pubmed.ncbi.nlm.nih.gov/21169367 21169367]|[https://pubmed.ncbi.nlm.nih.gov/25190359 25190359]|[https://pubmed.ncbi.nlm.nih.gov/27928741 27928741]|[https://pubmed.ncbi.nlm.nih.gov/22496646 22496646]|[https://pubmed.ncbi.nlm.nih.gov/27384337 27384337]|[https://pubmed.ncbi.nlm.nih.gov/16897177 16897177]|[https://pubmed.ncbi.nlm.nih.gov/15228383 15228383]|[https://pubmed.ncbi.nlm.nih.gov/27379103 27379103]|[https://pubmed.ncbi.nlm.nih.gov/27189951 27189951]|[https://pubmed.ncbi.nlm.nih.gov/23475714 23475714]|[https://pubmed.ncbi.nlm.nih.gov/24907509 24907509]|[https://pubmed.ncbi.nlm.nih.gov/20558211 20558211]|[https://pubmed.ncbi.nlm.nih.gov/28060516 28060516]|[https://pubmed.ncbi.nlm.nih.gov/20441997 20441997]|[https://pubmed.ncbi.nlm.nih.gov/27720757 27720757]|[https://pubmed.ncbi.nlm.nih.gov/28817611 28817611]|[https://pubmed.ncbi.nlm.nih.gov/24550396 24550396]|[https://pubmed.ncbi.nlm.nih.gov/19494052 19494052]|[https://pubmed.ncbi.nlm.nih.gov/19515361 19515361]| | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/29475940 29475940]|[https://pubmed.ncbi.nlm.nih.gov/29803109 29803109]|[https://pubmed.ncbi.nlm.nih.gov/12603202 12603202]|[https://pubmed.ncbi.nlm.nih.gov/31817246 31817246]|[https://pubmed.ncbi.nlm.nih.gov/31719144 31719144]|[https://pubmed.ncbi.nlm.nih.gov/21057214 21057214]|[https://pubmed.ncbi.nlm.nih.gov/31932305 31932305]|[https://pubmed.ncbi.nlm.nih.gov/17132514 17132514]|[https://pubmed.ncbi.nlm.nih.gov/30382146 30382146]|[https://pubmed.ncbi.nlm.nih.gov/27731363 27731363]|[https://pubmed.ncbi.nlm.nih.gov/31325506 31325506]|[https://pubmed.ncbi.nlm.nih.gov/25746926 25746926]|[https://pubmed.ncbi.nlm.nih.gov/26513758 26513758]|[https://pubmed.ncbi.nlm.nih.gov/16522637 16522637]|[https://pubmed.ncbi.nlm.nih.gov/21169367 21169367]|[https://pubmed.ncbi.nlm.nih.gov/25190359 25190359]|[https://pubmed.ncbi.nlm.nih.gov/27928741 27928741]|[https://pubmed.ncbi.nlm.nih.gov/22496646 22496646]|[https://pubmed.ncbi.nlm.nih.gov/27384337 27384337]|[https://pubmed.ncbi.nlm.nih.gov/16897177 16897177]|[https://pubmed.ncbi.nlm.nih.gov/15228383 15228383]|[https://pubmed.ncbi.nlm.nih.gov/27379103 27379103]|[https://pubmed.ncbi.nlm.nih.gov/27189951 27189951]|[https://pubmed.ncbi.nlm.nih.gov/23475714 23475714]|[https://pubmed.ncbi.nlm.nih.gov/24907509 24907509]|[https://pubmed.ncbi.nlm.nih.gov/20558211 20558211]|[https://pubmed.ncbi.nlm.nih.gov/28060516 28060516]|[https://pubmed.ncbi.nlm.nih.gov/20441997 20441997]|[https://pubmed.ncbi.nlm.nih.gov/27720757 27720757]|[https://pubmed.ncbi.nlm.nih.gov/28817611 28817611]|[https://pubmed.ncbi.nlm.nih.gov/24550396 24550396]|[https://pubmed.ncbi.nlm.nih.gov/19494052 19494052]|[https://pubmed.ncbi.nlm.nih.gov/19515361 19515361]|[https://pubmed.ncbi.nlm.nih.gov/28630087 28630087]|[https://pubmed.ncbi.nlm.nih.gov/31320997 31320997]|[https://pubmed.ncbi.nlm.nih.gov/30651366 30651366]|[https://pubmed.ncbi.nlm.nih.gov/30253927 30253927]|[https://pubmed.ncbi.nlm.nih.gov/25922361 25922361]|[https://pubmed.ncbi.nlm.nih.gov/31655162 31655162]|[https://pubmed.ncbi.nlm.nih.gov/18056652 18056652]|[https://pubmed.ncbi.nlm.nih.gov/24467287 24467287]|[https://pubmed.ncbi.nlm.nih.gov/32367383 32367383] <br> | ||
'''definition''' : Any N-linked glycan derivative with composition Man1-4Fuc0-1 GlcNAc2, in which the chitobiose core (GlcNAc2) is intact. Found in relatively large amounts in invertebrates and plants, they are formed by removal of the non-reducing terminal GlcNAc (\added by GlcNAcT1) from GlcNAcMan3-5GlcNAc2 by a Golgi hexosaminidase and subsequent removal of Man residues by Golgi mannosidases. Pauscimannose glycans have also been detected in vertebrates.[CHEBI:144386] <br> | '''definition''' : Any N-linked glycan derivative with composition Man1-4Fuc0-1 GlcNAc2, in which the chitobiose core (GlcNAc2) is intact. Found in relatively large amounts in invertebrates and plants, they are formed by removal of the non-reducing terminal GlcNAc (\added by GlcNAcT1) from GlcNAcMan3-5GlcNAc2 by a Golgi hexosaminidase and subsequent removal of Man residues by Golgi mannosidases. Pauscimannose glycans have also been detected in vertebrates.[CHEBI:144386] <br> | ||
'''term_xref''' : CHEBI:144386 <br> | '''term_xref''' : CHEBI:144386 <br> | ||
| Line 382: | Line 382: | ||
'''definition''' : A branched amino tetrasaccharide comprised of a trisaccharide chain of N-acetyl-α-neuraminic acid, β-D-galactose and N-acetyl-β-D-glucosamine residues linked sequentially (2→3) and (1→4), to the galactose residue of which is also linked (1→4) an N-acetyl-β-L-galactosamine residue. | '''definition''' : A branched amino tetrasaccharide comprised of a trisaccharide chain of N-acetyl-α-neuraminic acid, β-D-galactose and N-acetyl-β-D-glucosamine residues linked sequentially (2→3) and (1→4), to the galactose residue of which is also linked (1→4) an N-acetyl-β-L-galactosamine residue. | ||
[CHEBI:71561] <br> | [CHEBI:71561] <br> | ||
'''term_xref''' : GlycoMotif:GGM.000051|GTC:G41052GC|CID: | '''term_xref''' : GlycoMotif:GGM.000051|GTC:G41052GC|CID:70698375|CHEBI:71561 <br> | ||
'''synonyms''' : Sd^a /Cad <br> | '''synonyms''' : Sd^a /Cad <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
| Line 398: | Line 398: | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/30226570 30226570]|[https://pubmed.ncbi.nlm.nih.gov/3341453 3341453]|[https://pubmed.ncbi.nlm.nih.gov/12725333 12725333] <br> | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/30226570 30226570]|[https://pubmed.ncbi.nlm.nih.gov/3341453 3341453]|[https://pubmed.ncbi.nlm.nih.gov/12725333 12725333] <br> | ||
'''definition''' : (2S,4S,5R,6R)-5-Acetamido-2-[(2S,3R,4S,5S,6R)-2-[(2R,3S,4R,5R,6S)-5-acetamido-6-[(2S,3R,4S,5S,6R)-2-[(2R,3S,4R,5R,6R)-5-acetamido-6-hydroxy-2-(hydroxymethyl)-4-[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-3-yl]oxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-(hydroxymethyl)-4-[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-3-yl]oxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid is a member of neuraminic acids.[CHEBI:148203] <br> | '''definition''' : (2S,4S,5R,6R)-5-Acetamido-2-[(2S,3R,4S,5S,6R)-2-[(2R,3S,4R,5R,6S)-5-acetamido-6-[(2S,3R,4S,5S,6R)-2-[(2R,3S,4R,5R,6R)-5-acetamido-6-hydroxy-2-(hydroxymethyl)-4-[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-3-yl]oxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-(hydroxymethyl)-4-[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-3-yl]oxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid is a member of neuraminic acids.[CHEBI:148203] <br> | ||
'''term_xref''' : GlycoMotif:GGM.000032|GTC:G19619KS|CID: | '''term_xref''' : GlycoMotif:GGM.000032|GTC:G19619KS|CID:91856705|CHEBI:148203 <br> | ||
'''synonyms''' : Sdlex|SDLe^x|SDLex <br> | '''synonyms''' : Sdlex|SDLe^x|SDLex <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
| Line 412: | Line 412: | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G72548RZ G72548RZ] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G72548RZ G72548RZ] <br> | ||
'''term_in_sentence''' : A concise total synthesis of seminolipid, a sulfoglycolipid, has been achieved; key features include regioselective, tin-free sulfation of allyl β-d-galactopyranoside using 2,6-bis(trifluoromethyl)phenylboronic acid as protective reagent, stereoselective epoxidation, and site-selective acylation. [PMID: [https://pubmed.ncbi.nlm.nih.gov/31353379 31353379]] <br> | '''term_in_sentence''' : A concise total synthesis of seminolipid, a sulfoglycolipid, has been achieved; key features include regioselective, tin-free sulfation of allyl β-d-galactopyranoside using 2,6-bis(trifluoromethyl)phenylboronic acid as protective reagent, stereoselective epoxidation, and site-selective acylation. [PMID: [https://pubmed.ncbi.nlm.nih.gov/31353379 31353379]] <br> | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/31353379 31353379]|[https://pubmed.ncbi.nlm.nih.gov/31736776 31736776]|[https://pubmed.ncbi.nlm.nih.gov/12810822 12810822]|[https://pubmed.ncbi.nlm.nih.gov/19582571 19582571]|[https://pubmed.ncbi.nlm.nih.gov/19542524 19542524]|[https://pubmed.ncbi.nlm.nih.gov/20670037 20670037]|[https://pubmed.ncbi.nlm.nih.gov/15659616 15659616]|[https://pubmed.ncbi.nlm.nih.gov/20817833 20817833]|870059|[https://pubmed.ncbi.nlm.nih.gov/10801776 10801776]|[https://pubmed.ncbi.nlm.nih.gov/21965315 21965315]|[https://pubmed.ncbi.nlm.nih.gov/8095813 8095813]|[https://pubmed.ncbi.nlm.nih.gov/1280685 1280685]|[https://pubmed.ncbi.nlm.nih.gov/4690236 4690236]|[https://pubmed.ncbi.nlm.nih.gov/34199863 34199863]|[https://pubmed.ncbi.nlm.nih.gov/4207869 4207869]|[https://pubmed.ncbi.nlm.nih.gov/2373957 2373957]|845131 <br> | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/31353379 31353379]|[https://pubmed.ncbi.nlm.nih.gov/31736776 31736776]|[https://pubmed.ncbi.nlm.nih.gov/12810822 12810822]|[https://pubmed.ncbi.nlm.nih.gov/19582571 19582571]|[https://pubmed.ncbi.nlm.nih.gov/19542524 19542524]|[https://pubmed.ncbi.nlm.nih.gov/20670037 20670037]|[https://pubmed.ncbi.nlm.nih.gov/15659616 15659616]|[https://pubmed.ncbi.nlm.nih.gov/20817833 20817833]|[https://pubmed.ncbi.nlm.nih.gov/870059 870059]|[https://pubmed.ncbi.nlm.nih.gov/10801776 10801776]|[https://pubmed.ncbi.nlm.nih.gov/21965315 21965315]|[https://pubmed.ncbi.nlm.nih.gov/8095813 8095813]|[https://pubmed.ncbi.nlm.nih.gov/1280685 1280685]|[https://pubmed.ncbi.nlm.nih.gov/4690236 4690236]|[https://pubmed.ncbi.nlm.nih.gov/34199863 34199863]|[https://pubmed.ncbi.nlm.nih.gov/4207869 4207869]|[https://pubmed.ncbi.nlm.nih.gov/2373957 2373957]|[https://pubmed.ncbi.nlm.nih.gov/845131 845131] <br> | ||
'''definition''' : <br> | '''definition''' : <br> | ||
'''term_xref''' : GlycoMotif:GGM.000062|GTC:G72548RZ<br> | '''term_xref''' : GlycoMotif:GGM.000062|GTC:G72548RZ<br> | ||
| Line 430: | Line 430: | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/2139874 2139874] <br> | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/2139874 2139874] <br> | ||
'''definition''' : A ganglioside in which the oligosaccharide portion is composed of a pentasaccharide containing one or more sialic acid residues.[CHEBI:36542] <br> | '''definition''' : A ganglioside in which the oligosaccharide portion is composed of a pentasaccharide containing one or more sialic acid residues.[CHEBI:36542] <br> | ||
'''term_xref''' : GlycoMotif:GGM.000097|GTC:G13224CR|CID: | '''term_xref''' : GlycoMotif:GGM.000097|GTC:G13224CR|CID: 91859576|CHEBI:148045|KEGG:G00883|CHEBI:36542 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
| Line 446: | Line 446: | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/8862128 8862128]|[https://pubmed.ncbi.nlm.nih.gov/7236652 7236652]|[https://pubmed.ncbi.nlm.nih.gov/2370108 2370108]|[https://pubmed.ncbi.nlm.nih.gov/3133978 3133978]|[https://pubmed.ncbi.nlm.nih.gov/2905604 2905604]|[https://pubmed.ncbi.nlm.nih.gov/1749114 1749114]|[https://pubmed.ncbi.nlm.nih.gov/1573418 1573418] <br> | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/8862128 8862128]|[https://pubmed.ncbi.nlm.nih.gov/7236652 7236652]|[https://pubmed.ncbi.nlm.nih.gov/2370108 2370108]|[https://pubmed.ncbi.nlm.nih.gov/3133978 3133978]|[https://pubmed.ncbi.nlm.nih.gov/2905604 2905604]|[https://pubmed.ncbi.nlm.nih.gov/1749114 1749114]|[https://pubmed.ncbi.nlm.nih.gov/1573418 1573418] <br> | ||
'''definition''' : α-Neup5Ac-(2→3)-β-D-Galp-(1→4)-D-GlcpNAc with β configuration at the anomeric position of the reducing-end N-acetyl-D-glucosamine residue.[CHEBI:71612] <br> | '''definition''' : α-Neup5Ac-(2→3)-β-D-Galp-(1→4)-D-GlcpNAc with β configuration at the anomeric position of the reducing-end N-acetyl-D-glucosamine residue.[CHEBI:71612] <br> | ||
'''term_xref''' : GlycoMotif:GGM.000081|GTC:G50123ZO|CID: | '''term_xref''' : GlycoMotif:GGM.000081|GTC:G50123ZO|CID:91845504|KEGG:G01429|CHEBI:151914 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
| Line 459: | Line 459: | ||
'''glycan_dictionary_accession''' : GSD000150 <br> | '''glycan_dictionary_accession''' : GSD000150 <br> | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G00053MO G00053MO] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G00053MO G00053MO] <br> | ||
'''term_in_sentence''' : By comparing the serum O-glycan profiles from healthy controls with those of cancer patients, we identified a marker candidate, core 1 sialyl Lewis A (NeuAcα2-3Galβ1-3(Fucα1-4)GlcNAcβ1-3Gal) (abbreviated C1SLA), whose concentration appeared to be weakly correlated with CA19-9 values.[PMID: | '''term_in_sentence''' : By comparing the serum O-glycan profiles from healthy controls with those of cancer patients, we identified a marker candidate, core 1 sialyl Lewis A (NeuAcα2-3Galβ1-3(Fucα1-4)GlcNAcβ1-3Gal) (abbreviated C1SLA), whose concentration appeared to be weakly correlated with CA19-9 values.[PMID:[https://pubmed.ncbi.nlm.nih.gov/26641888 26641888]] <br> | ||
'''publication''' : | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/26641888 26641888]|[https://pubmed.ncbi.nlm.nih.gov/31424423 31424423]|[https://pubmed.ncbi.nlm.nih.gov/26530370 26530370]|[https://pubmed.ncbi.nlm.nih.gov/17760270 17760270]|[https://pubmed.ncbi.nlm.nih.gov/18343410 18343410]|[https://pubmed.ncbi.nlm.nih.gov/16033091 16033091]|[https://pubmed.ncbi.nlm.nih.gov/8829184 8829184]|[https://pubmed.ncbi.nlm.nih.gov/11907351 11907351]|[https://pubmed.ncbi.nlm.nih.gov/20592391 20592391]|[https://pubmed.ncbi.nlm.nih.gov/26641888 26641888]|[https://pubmed.ncbi.nlm.nih.gov/24128856 24128856]|[https://pubmed.ncbi.nlm.nih.gov/30249386 30249386]|[https://pubmed.ncbi.nlm.nih.gov/10773785 10773785]|[https://pubmed.ncbi.nlm.nih.gov/16149606 16149606]|[https://pubmed.ncbi.nlm.nih.gov/10996727 10996727]|[https://pubmed.ncbi.nlm.nih.gov/10436809 10436809]|[https://pubmed.ncbi.nlm.nih.gov/10667238 10667238]|[https://pubmed.ncbi.nlm.nih.gov/7720892 7720892]|[https://pubmed.ncbi.nlm.nih.gov/7630017 7630017]|[https://pubmed.ncbi.nlm.nih.gov/10612415 10612415]|[https://pubmed.ncbi.nlm.nih.gov/9836451 9836451]|[https://pubmed.ncbi.nlm.nih.gov/12511721 12511721]|[https://pubmed.ncbi.nlm.nih.gov/22623153 22623153] <br> | ||
'''definition''' : A sialyated version of Lewis A with a branched structure of amino tetrasaccharide consisting of a sialyl residue, linked (2→3) to a galactosyl residue that in turn is linked (1→3) to a glucosaminyl residue at the reducing end, which is also carrying a fucosyl residue at the 4-position.[CHEBI:62681] <br> | '''definition''' : A sialyated version of Lewis A with a branched structure of amino tetrasaccharide consisting of a sialyl residue, linked (2→3) to a galactosyl residue that in turn is linked (1→3) to a glucosaminyl residue at the reducing end, which is also carrying a fucosyl residue at the 4-position.[CHEBI:62681] <br> | ||
'''term_xref''' : GTC:G00053MO|GlycoEpitope:EP0008|CID: | '''term_xref''' : GTC:G00053MO|GlycoEpitope:EP0008|CID:53356732|CHEBI:62681|GlycoMotif:GGM.000024<br> | ||
'''synonyms''' : SLea|CA19-9|CA19-9 antigen|SLe^a|Slea <br> | '''synonyms''' : SLea|CA19-9|CA19-9 antigen|SLe^a|Slea <br> | ||
'''function''' : The carbohydrate determinants,Sialyl Lea and Sialyl Lex,which are frequently expressed on human cancer cells,serve as ligands for a cell adhesion molecule of the selectin family,E-selectin,which is expressed on vascular endothelial cells. These carbohydrate determinants are involved in the adhesion of cancer cells to vascular endothelium and thus contribute to hematogenous metastasis of cancer.[GlycoEpitope:EP0008] <br> | '''function''' : The carbohydrate determinants,Sialyl Lea and Sialyl Lex,which are frequently expressed on human cancer cells,serve as ligands for a cell adhesion molecule of the selectin family,E-selectin,which is expressed on vascular endothelial cells. These carbohydrate determinants are involved in the adhesion of cancer cells to vascular endothelium and thus contribute to hematogenous metastasis of cancer.[GlycoEpitope:EP0008] <br> | ||
| Line 476: | Line 476: | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G00054MO G00054MO] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G00054MO G00054MO] <br> | ||
'''term_in_sentence''' : As sialyl Lewis X is a lignad of the selectin family, it has been proposed that sialyl Lewis X-rich colon cancer cells metastasize to the liver by adhesion to selectins on hepatic endothelial cells.[PMID:[https://pubmed.ncbi.nlm.nih.gov/12828079 12828079]] <br> | '''term_in_sentence''' : As sialyl Lewis X is a lignad of the selectin family, it has been proposed that sialyl Lewis X-rich colon cancer cells metastasize to the liver by adhesion to selectins on hepatic endothelial cells.[PMID:[https://pubmed.ncbi.nlm.nih.gov/12828079 12828079]] <br> | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/12828079 12828079]| | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/12828079 12828079]|[https://pubmed.ncbi.nlm.nih.gov/8940172 8940172]|[https://pubmed.ncbi.nlm.nih.gov/10075923 10075923]|[https://pubmed.ncbi.nlm.nih.gov/19054801 19054801]|[https://pubmed.ncbi.nlm.nih.gov/9642161 9642161]|[https://pubmed.ncbi.nlm.nih.gov/31054033 31054033]|[https://pubmed.ncbi.nlm.nih.gov/15229399 15229399]|[https://pubmed.ncbi.nlm.nih.gov/29061785 29061785]|[https://pubmed.ncbi.nlm.nih.gov/16033070 16033070]|[https://pubmed.ncbi.nlm.nih.gov/10728707 10728707]|[https://pubmed.ncbi.nlm.nih.gov/9556613 9556613]|[https://pubmed.ncbi.nlm.nih.gov/8940172 8940172]|[https://pubmed.ncbi.nlm.nih.gov/9298692 9298692]|[https://pubmed.ncbi.nlm.nih.gov/18607721 18607721]|[https://pubmed.ncbi.nlm.nih.gov/22593503 22593503]|[https://pubmed.ncbi.nlm.nih.gov/25944902 25944902]| [https://pubmed.ncbi.nlm.nih.gov/9642161 9642161]|[https://pubmed.ncbi.nlm.nih.gov/15780091 15780091]|[https://pubmed.ncbi.nlm.nih.gov/20401888 20401888] <br> | ||
'''definition''' : A sialylated version of Lewis X antigen expressed on cell surfaces with structure alpha-Neup5Ac-(2->3)-beta-D-Galp-(1->4)-[alpha-L-Fucp-(1->3)]-beta-D-GlcpNAcis α-Neup5Ac-(2→3)-β-D-Galp-(1→4)-[α-L-Fucp-(1→3)]-D-GlcpNAc in which the anomeric configuration of the reducing-end N-acetyl-beta-D-glucosamine residue is beta. It is a ligand for selectins. [MESH: | '''definition''' : A sialylated version of Lewis X antigen expressed on cell surfaces with structure alpha-Neup5Ac-(2->3)-beta-D-Galp-(1->4)-[alpha-L-Fucp-(1->3)]-beta-D-GlcpNAcis α-Neup5Ac-(2→3)-β-D-Galp-(1→4)-[α-L-Fucp-(1→3)]-D-GlcpNAc in which the anomeric configuration of the reducing-end N-acetyl-beta-D-glucosamine residue is beta. It is a ligand for selectins. [MESH:2030970],CHEI:71622 <br> | ||
'''term_xref''' : GTC:G00054MO |GlycoEpitope:EP0012|CID: | '''term_xref''' : GTC:G00054MO |GlycoEpitope:EP0012|CID: 44456859|CHEBI:71622|MESH:2030970|GlycoMotif:GGM.000022 <br> | ||
'''synonyms''' : SLe(x)|SLe^x|SLex|Slex <br> | '''synonyms''' : SLe(x)|SLe^x|SLex|Slex <br> | ||
'''function''' : Sialyl Lex was shown to be a specific ligand on human leukocytes for vascular E- and P-selectins,and was shown to mediate leukocyte recruitment.[GlycoEpitope:EP0012]|The determinant is also frequently expressed on human cancer cells,and is involved in the adhesion of cancer cells to vascular endothelium and thus contribute to hematogenous metastasis of cancer.[GlycoEpitope:EP0012] <br> | '''function''' : Sialyl Lex was shown to be a specific ligand on human leukocytes for vascular E- and P-selectins,and was shown to mediate leukocyte recruitment.[GlycoEpitope:EP0012]|The determinant is also frequently expressed on human cancer cells,and is involved in the adhesion of cancer cells to vascular endothelium and thus contribute to hematogenous metastasis of cancer.[GlycoEpitope:EP0012] <br> | ||
| Line 494: | Line 494: | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/9815711 9815711]|[https://pubmed.ncbi.nlm.nih.gov/9730796 9730796]|[https://pubmed.ncbi.nlm.nih.gov/11867833 11867833]|[https://pubmed.ncbi.nlm.nih.gov/9713476 9713476]|[https://pubmed.ncbi.nlm.nih.gov/2819663 2819663]|[https://pubmed.ncbi.nlm.nih.gov/9372327 9372327]|[https://pubmed.ncbi.nlm.nih.gov/9615762 9615762]|[https://pubmed.ncbi.nlm.nih.gov/1352835 1352835]|[https://pubmed.ncbi.nlm.nih.gov/2575680 2575680]|[https://pubmed.ncbi.nlm.nih.gov/9330526 9330526]|[https://pubmed.ncbi.nlm.nih.gov/8753037 8753037]|[https://pubmed.ncbi.nlm.nih.gov/2210805 2210805]|[https://pubmed.ncbi.nlm.nih.gov/12680155 12680155]|[https://pubmed.ncbi.nlm.nih.gov/11974864 11974864]|[https://pubmed.ncbi.nlm.nih.gov/10453898 10453898]|[https://pubmed.ncbi.nlm.nih.gov/2565704 2565704]|[https://pubmed.ncbi.nlm.nih.gov/17144590 17144590] <br> | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/9815711 9815711]|[https://pubmed.ncbi.nlm.nih.gov/9730796 9730796]|[https://pubmed.ncbi.nlm.nih.gov/11867833 11867833]|[https://pubmed.ncbi.nlm.nih.gov/9713476 9713476]|[https://pubmed.ncbi.nlm.nih.gov/2819663 2819663]|[https://pubmed.ncbi.nlm.nih.gov/9372327 9372327]|[https://pubmed.ncbi.nlm.nih.gov/9615762 9615762]|[https://pubmed.ncbi.nlm.nih.gov/1352835 1352835]|[https://pubmed.ncbi.nlm.nih.gov/2575680 2575680]|[https://pubmed.ncbi.nlm.nih.gov/9330526 9330526]|[https://pubmed.ncbi.nlm.nih.gov/8753037 8753037]|[https://pubmed.ncbi.nlm.nih.gov/2210805 2210805]|[https://pubmed.ncbi.nlm.nih.gov/12680155 12680155]|[https://pubmed.ncbi.nlm.nih.gov/11974864 11974864]|[https://pubmed.ncbi.nlm.nih.gov/10453898 10453898]|[https://pubmed.ncbi.nlm.nih.gov/2565704 2565704]|[https://pubmed.ncbi.nlm.nih.gov/17144590 17144590] <br> | ||
'''definition''' : <br> | '''definition''' : <br> | ||
'''term_xref''' : GTC:G06597DS|GlycoEpitope:EP0043|CID: | '''term_xref''' : GTC:G06597DS|GlycoEpitope:EP0043|CID: 91855346|CHEBI:147019 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : hematogenous metastasis[GlycoEpitope:EP0043]|the progression of inflammation[GlycoEpitope:EP0043] <br> | '''function''' : hematogenous metastasis[GlycoEpitope:EP0043]|the progression of inflammation[GlycoEpitope:EP0043] <br> | ||
| Line 508: | Line 508: | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G65562ZE G65562ZE] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G65562ZE G65562ZE] <br> | ||
'''term_in_sentence''' : The expression and functional analysis of the sialyl-T antigen in prostate cancer.[PMID:[https://pubmed.ncbi.nlm.nih.gov/32583304 32583304]] <br> | '''term_in_sentence''' : The expression and functional analysis of the sialyl-T antigen in prostate cancer.[PMID:[https://pubmed.ncbi.nlm.nih.gov/32583304 32583304]] <br> | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/32583304 32583304]|[https://pubmed.ncbi.nlm.nih.gov/31897478 31897478]|[https://pubmed.ncbi.nlm.nih.gov/31719620 31719620]| | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/32583304 32583304]|[https://pubmed.ncbi.nlm.nih.gov/31897478 31897478]|[https://pubmed.ncbi.nlm.nih.gov/31719620 31719620]| [https://pubmed.ncbi.nlm.nih.gov/10365852 10365852]|[https://pubmed.ncbi.nlm.nih.gov/25727149 25727149]|[https://pubmed.ncbi.nlm.nih.gov/11523928 11523928]|[https://pubmed.ncbi.nlm.nih.gov/20407596 20407596]|[https://pubmed.ncbi.nlm.nih.gov/25464017 25464017]|[https://pubmed.ncbi.nlm.nih.gov/30864133 30864133]|[https://pubmed.ncbi.nlm.nih.gov/26391208 26391208]|[https://pubmed.ncbi.nlm.nih.gov/19811634 19811634]|[https://pubmed.ncbi.nlm.nih.gov/29796594 29796594]|[https://pubmed.ncbi.nlm.nih.gov/23725156 23725156]|[https://pubmed.ncbi.nlm.nih.gov/8905873 8905873]|[https://pubmed.ncbi.nlm.nih.gov/11192238 11192238]|[https://pubmed.ncbi.nlm.nih.gov/18275089 18275089]|[https://pubmed.ncbi.nlm.nih.gov/27037304 27037304]|[https://pubmed.ncbi.nlm.nih.gov/11949946 11949946]|[https://pubmed.ncbi.nlm.nih.gov/15604091 15604091]|[https://pubmed.ncbi.nlm.nih.gov/24118321 24118321] <br> | ||
'''definition''' : Alpha-Neup5Ac-(2→3)-beta-D-Galp-(1→3)-alpha-D-GalpNAc is alpha-Neup5Ac-(2→3)-beta-D-Galp-(1→3)-D-GalpNAc in which the configuration at the GalNAc anomeric carbon is alpha. It has a role as an epitope.[CHEBI:65257] <br> | '''definition''' : Alpha-Neup5Ac-(2→3)-beta-D-Galp-(1→3)-alpha-D-GalpNAc is alpha-Neup5Ac-(2→3)-beta-D-Galp-(1→3)-D-GalpNAc in which the configuration at the GalNAc anomeric carbon is alpha. It has a role as an epitope.[CHEBI:65257] <br> | ||
'''term_xref''' : GlycoMotif:GGM.000013|GTC:G65562ZE|CID: | '''term_xref''' : GlycoMotif:GGM.000013|GTC:G65562ZE|CID:44611726|CHEBI:65257 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
| Line 585: | Line 585: | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G36123IU G36123IU] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G36123IU G36123IU] <br> | ||
'''term_in_sentence''' : The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-β secretion from monocytes/macrophages through the DAP12-Syk pathway.[PMID:[https://pubmed.ncbi.nlm.nih.gov/23035012 23035012]] <br> | '''term_in_sentence''' : The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-β secretion from monocytes/macrophages through the DAP12-Syk pathway.[PMID:[https://pubmed.ncbi.nlm.nih.gov/23035012 23035012]] <br> | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/23035012 23035012]|[https://pubmed.ncbi.nlm.nih.gov/29273237 29273237]|[https://pubmed.ncbi.nlm.nih.gov/15770530 15770530]|[https://pubmed.ncbi.nlm.nih.gov/31595388 31595388]|[https://pubmed.ncbi.nlm.nih.gov/26617889 26617889]|[https://pubmed.ncbi.nlm.nih.gov/1720922 1720922]|[https://pubmed.ncbi.nlm.nih.gov/15466199 15466199]|[https://pubmed.ncbi.nlm.nih.gov/10971172 10971172]|[https://pubmed.ncbi.nlm.nih.gov/14652017 14652017]|[https://pubmed.ncbi.nlm.nih.gov/8616877 8616877]|[https://pubmed.ncbi.nlm.nih.gov/1543361 1543361]|[https://pubmed.ncbi.nlm.nih.gov/16965854 16965854]|[https://pubmed.ncbi.nlm.nih.gov/21566901 21566901]|[https://pubmed.ncbi.nlm.nih.gov/9269833 9269833]|[https://pubmed.ncbi.nlm.nih.gov/9834267 9834267]|[https://pubmed.ncbi.nlm.nih.gov/24970145 24970145]|[https://pubmed.ncbi.nlm.nih.gov/22367369 22367369]|[https://pubmed.ncbi.nlm.nih.gov/10778170 10778170]|[https://pubmed.ncbi.nlm.nih.gov/30018351 30018351]|[https://pubmed.ncbi.nlm.nih.gov/28628803 28628803]|[https://pubmed.ncbi.nlm.nih.gov/16149605 16149605]|[https://pubmed.ncbi.nlm.nih.gov/32134186 32134186]|[https://pubmed.ncbi.nlm.nih.gov/28632300 28632300]|[https://pubmed.ncbi.nlm.nih.gov/11308020 11308020]| | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/23035012 23035012]|[https://pubmed.ncbi.nlm.nih.gov/29273237 29273237]|[https://pubmed.ncbi.nlm.nih.gov/15770530 15770530]|[https://pubmed.ncbi.nlm.nih.gov/31595388 31595388]|[https://pubmed.ncbi.nlm.nih.gov/26617889 26617889]|[https://pubmed.ncbi.nlm.nih.gov/1720922 1720922]|[https://pubmed.ncbi.nlm.nih.gov/15466199 15466199]|[https://pubmed.ncbi.nlm.nih.gov/10971172 10971172]|[https://pubmed.ncbi.nlm.nih.gov/14652017 14652017]|[https://pubmed.ncbi.nlm.nih.gov/8616877 8616877]|[https://pubmed.ncbi.nlm.nih.gov/1543361 1543361]|[https://pubmed.ncbi.nlm.nih.gov/16965854 16965854]|[https://pubmed.ncbi.nlm.nih.gov/21566901 21566901]|[https://pubmed.ncbi.nlm.nih.gov/9269833 9269833]|[https://pubmed.ncbi.nlm.nih.gov/9834267 9834267]|[https://pubmed.ncbi.nlm.nih.gov/24970145 24970145]|[https://pubmed.ncbi.nlm.nih.gov/22367369 22367369]|[https://pubmed.ncbi.nlm.nih.gov/10778170 10778170]|[https://pubmed.ncbi.nlm.nih.gov/30018351 30018351]|[https://pubmed.ncbi.nlm.nih.gov/28628803 28628803]|[https://pubmed.ncbi.nlm.nih.gov/16149605 16149605]|[https://pubmed.ncbi.nlm.nih.gov/32134186 32134186]|[https://pubmed.ncbi.nlm.nih.gov/28632300 28632300]|[https://pubmed.ncbi.nlm.nih.gov/11308020 11308020]| [https://pubmed.ncbi.nlm.nih.gov/10365852 10365852]|[https://pubmed.ncbi.nlm.nih.gov/17195456 17195456]|[https://pubmed.ncbi.nlm.nih.gov/23567325 23567325]|[https://pubmed.ncbi.nlm.nih.gov/11014575 11014575]|[https://pubmed.ncbi.nlm.nih.gov/2342728 2342728]|[https://pubmed.ncbi.nlm.nih.gov/8575847 8575847]|[https://pubmed.ncbi.nlm.nih.gov/1851822 1851822]|[https://pubmed.ncbi.nlm.nih.gov/11735169 11735169]|[https://pubmed.ncbi.nlm.nih.gov/20960853 20960853]|[https://pubmed.ncbi.nlm.nih.gov/7846011 7846011] <br> | ||
'''definition''' : N-acetyl-alpha-neuraminyl-(2→6)-N-acetyl-alpha-D-galactosamine is an N-acetyl-alpha-neuraminyl-(2→6)-N-acetyl-D-galactosamine in which the carbon bearing the anomeric hydroxy group has alpha configuration. It has a role as an epitope. It derives from a N-acetyl-alpha-D-galactosamine and a N-acetyl-alpha-neuraminic acid.[CHEBI:61818] <br> | '''definition''' : N-acetyl-alpha-neuraminyl-(2→6)-N-acetyl-alpha-D-galactosamine is an N-acetyl-alpha-neuraminyl-(2→6)-N-acetyl-D-galactosamine in which the carbon bearing the anomeric hydroxy group has alpha configuration. It has a role as an epitope. It derives from a N-acetyl-alpha-D-galactosamine and a N-acetyl-alpha-neuraminic acid.[CHEBI:61818] <br> | ||
'''term_xref''' : GTC:G36123IU|GlycoEpitope:EP0022|CID: | '''term_xref''' : GTC:G36123IU|GlycoEpitope:EP0022|CID:51351800|CHEBI:61818 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : apoptosis of monocyte-derived dendritic cells[GlycoEpitope:EP0022]|decrease of adhesion and increase of migration[GlycoEpitope:EP0022]|increase of tumor growth[GlycoEpitope:EP0022]|associated with morphological changes, decreased growth and increased migration of MDA-MB-231 cells.[GlycoEpitope:EP0022] <br> | '''function''' : apoptosis of monocyte-derived dendritic cells[GlycoEpitope:EP0022]|decrease of adhesion and increase of migration[GlycoEpitope:EP0022]|increase of tumor growth[GlycoEpitope:EP0022]|associated with morphological changes, decreased growth and increased migration of MDA-MB-231 cells.[GlycoEpitope:EP0022] <br> | ||
| Line 616: | Line 616: | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G85497PI G85497PI] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G85497PI G85497PI] <br> | ||
'''term_in_sentence''' : Our findings suggest that expression of SSEA-1 immunoreactivity in thyroid neoplasms was associated with more aggressive thyroid carcinomas.[PMID:[https://pubmed.ncbi.nlm.nih.gov/27550342 27550342]] <br> | '''term_in_sentence''' : Our findings suggest that expression of SSEA-1 immunoreactivity in thyroid neoplasms was associated with more aggressive thyroid carcinomas.[PMID:[https://pubmed.ncbi.nlm.nih.gov/27550342 27550342]] <br> | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/27550342 27550342]|[https://pubmed.ncbi.nlm.nih.gov/25564944 25564944]|[https://pubmed.ncbi.nlm.nih.gov/30987116 30987116]|[https://pubmed.ncbi.nlm.nih.gov/10778174 10778174]|[https://pubmed.ncbi.nlm.nih.gov/19427293 19427293]|[https://pubmed.ncbi.nlm.nih.gov/24923741 24923741]|[https://pubmed.ncbi.nlm.nih.gov/28092949 28092949]|[https://pubmed.ncbi.nlm.nih.gov/16499901 16499901]|[https://pubmed.ncbi.nlm.nih.gov/10470652 10470652]|[https://pubmed.ncbi.nlm.nih.gov/18787210 18787210]|[https://pubmed.ncbi.nlm.nih.gov/2885312 2885312]|[https://pubmed.ncbi.nlm.nih.gov/16149609 16149609]|[https://pubmed.ncbi.nlm.nih.gov/17003364 17003364]|[https://pubmed.ncbi.nlm.nih.gov/27363517 27363517]|[https://pubmed.ncbi.nlm.nih.gov/15505339 15505339]|[https://pubmed.ncbi.nlm.nih.gov/27990704 27990704]|[https://pubmed.ncbi.nlm.nih.gov/2568298 2568298]|[https://pubmed.ncbi.nlm.nih.gov/20491542 20491542]|[https://pubmed.ncbi.nlm.nih.gov/23572256 23572256]|[https://pubmed.ncbi.nlm.nih.gov/1977666 1977666]|[https://pubmed.ncbi.nlm.nih.gov/9553168 9553168]| | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/27550342 27550342]|[https://pubmed.ncbi.nlm.nih.gov/25564944 25564944]|[https://pubmed.ncbi.nlm.nih.gov/30987116 30987116]|[https://pubmed.ncbi.nlm.nih.gov/10778174 10778174]|[https://pubmed.ncbi.nlm.nih.gov/19427293 19427293]|[https://pubmed.ncbi.nlm.nih.gov/24923741 24923741]|[https://pubmed.ncbi.nlm.nih.gov/28092949 28092949]|[https://pubmed.ncbi.nlm.nih.gov/16499901 16499901]|[https://pubmed.ncbi.nlm.nih.gov/10470652 10470652]|[https://pubmed.ncbi.nlm.nih.gov/18787210 18787210]|[https://pubmed.ncbi.nlm.nih.gov/2885312 2885312]|[https://pubmed.ncbi.nlm.nih.gov/16149609 16149609]|[https://pubmed.ncbi.nlm.nih.gov/17003364 17003364]|[https://pubmed.ncbi.nlm.nih.gov/27363517 27363517]|[https://pubmed.ncbi.nlm.nih.gov/15505339 15505339]|[https://pubmed.ncbi.nlm.nih.gov/27990704 27990704]|[https://pubmed.ncbi.nlm.nih.gov/2568298 2568298]|[https://pubmed.ncbi.nlm.nih.gov/20491542 20491542]|[https://pubmed.ncbi.nlm.nih.gov/23572256 23572256]|[https://pubmed.ncbi.nlm.nih.gov/1977666 1977666]|[https://pubmed.ncbi.nlm.nih.gov/9553168 9553168]|[https://pubmed.ncbi.nlm.nih.gov/20458727 20458727]|[https://pubmed.ncbi.nlm.nih.gov/2566639 2566639]|[https://pubmed.ncbi.nlm.nih.gov/14577357 14577357]|[https://pubmed.ncbi.nlm.nih.gov/20960856 20960856]|[https://pubmed.ncbi.nlm.nih.gov/15547746 15547746]|[https://pubmed.ncbi.nlm.nih.gov/9096265 9096265]|[https://pubmed.ncbi.nlm.nih.gov/30639212 30639212]|[https://pubmed.ncbi.nlm.nih.gov/1357195 1357195]|[https://pubmed.ncbi.nlm.nih.gov/1359630 1359630]|[https://pubmed.ncbi.nlm.nih.gov/20181261 20181261]|[https://pubmed.ncbi.nlm.nih.gov/20508065 20508065]|[https://pubmed.ncbi.nlm.nih.gov/2907526 2907526]|[https://pubmed.ncbi.nlm.nih.gov/22624699 22624699]|[https://pubmed.ncbi.nlm.nih.gov/22988836 22988836]|[https://pubmed.ncbi.nlm.nih.gov/17183690 17183690]|[https://pubmed.ncbi.nlm.nih.gov/27188587 27188587]|[https://pubmed.ncbi.nlm.nih.gov/7908065 7908065]|[https://pubmed.ncbi.nlm.nih.gov/10400391 10400391]|[https://pubmed.ncbi.nlm.nih.gov/6137429 6137429]|[https://pubmed.ncbi.nlm.nih.gov/1979260 1979260]|[https://pubmed.ncbi.nlm.nih.gov/2898921 2898921]|[https://pubmed.ncbi.nlm.nih.gov/14736812 14736812]|[https://pubmed.ncbi.nlm.nih.gov/30919971 30919971] <br> | ||
'''definition''' : <br> | '''definition''' : <br> | ||
'''term_xref''' : GTC:G85497PI |GlycoEpitope:EP0042|CID: | '''term_xref''' : GTC:G85497PI |GlycoEpitope:EP0042|CID:91851144|CHEBI:154343 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : This determinant is specifically recognized by the antibody raised against stage-specific embryonic antigen-1 (SSEA-1)[GlycoEpitope:EP0042]. The SSEA-1 antibody requires Lex hapten carried by i-antigenic structure (e.g.,nor-nLc6Cer).[GlycoEpitope:EP0042] <br> | '''function''' : This determinant is specifically recognized by the antibody raised against stage-specific embryonic antigen-1 (SSEA-1)[GlycoEpitope:EP0042]. The SSEA-1 antibody requires Lex hapten carried by i-antigenic structure (e.g.,nor-nLc6Cer).[GlycoEpitope:EP0042] <br> | ||
| Line 631: | Line 631: | ||
'''glycan_dictionary_accession''' : GSD000160 <br> | '''glycan_dictionary_accession''' : GSD000160 <br> | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G25129BT G25129BT] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G25129BT G25129BT] <br> | ||
'''term_in_sentence''' : Glycosphingolipids (GSLs), such as the globo-series GSLs stage-specific embryonic antigen 3 (SSEA-3), SSEA-4, and Globo-H, are specifically expressed on pluripotent stem cells and cancer cells, and are known to be associated with various biological processes such as cell recognition, cell adhesion, and signal transduction.[PMID: | '''term_in_sentence''' : Glycosphingolipids (GSLs), such as the globo-series GSLs stage-specific embryonic antigen 3 (SSEA-3), SSEA-4, and Globo-H, are specifically expressed on pluripotent stem cells and cancer cells, and are known to be associated with various biological processes such as cell recognition, cell adhesion, and signal transduction.[PMID: [https://pubmed.ncbi.nlm.nih.gov/31817926 31817926]] <br> | ||
'''publication''' : | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/31817926 31817926]|[https://pubmed.ncbi.nlm.nih.gov/30271856 30271856]|[https://pubmed.ncbi.nlm.nih.gov/32684574 32684574]|[https://pubmed.ncbi.nlm.nih.gov/26677875 26677875]|[https://pubmed.ncbi.nlm.nih.gov/23175153 23175153]|[https://pubmed.ncbi.nlm.nih.gov/32525407 32525407]| [https://pubmed.ncbi.nlm.nih.gov/29227830 29227830]|[https://pubmed.ncbi.nlm.nih.gov/[https://pubmed.ncbi.nlm.nih.gov/18773292 18773292] [https://pubmed.ncbi.nlm.nih.gov/18773292 18773292]]|[https://pubmed.ncbi.nlm.nih.gov/25561682 25561682]|[https://pubmed.ncbi.nlm.nih.gov/10837462 10837462]|[https://pubmed.ncbi.nlm.nih.gov/2412924 2412924]|[https://pubmed.ncbi.nlm.nih.gov/[https://pubmed.ncbi.nlm.nih.gov/21149348 21149348] [https://pubmed.ncbi.nlm.nih.gov/21149348 21149348]]|[https://pubmed.ncbi.nlm.nih.gov/[https://pubmed.ncbi.nlm.nih.gov/17008424 17008424] [https://pubmed.ncbi.nlm.nih.gov/17008424 17008424]]|[https://pubmed.ncbi.nlm.nih.gov/[https://pubmed.ncbi.nlm.nih.gov/2874662 2874662] [https://pubmed.ncbi.nlm.nih.gov/2874662 2874662]]|[https://pubmed.ncbi.nlm.nih.gov/25171543 25171543]|[https://pubmed.ncbi.nlm.nih.gov/31304790 31304790]|[https://pubmed.ncbi.nlm.nih.gov/31642560 31642560]|[https://pubmed.ncbi.nlm.nih.gov/30484221 30484221]|[https://pubmed.ncbi.nlm.nih.gov/30525067 30525067]|[https://pubmed.ncbi.nlm.nih.gov/21161592 21161592]|[https://pubmed.ncbi.nlm.nih.gov/27325407 27325407]|[https://pubmed.ncbi.nlm.nih.gov/28170296 28170296]|[https://pubmed.ncbi.nlm.nih.gov/29637816 29637816]| [https://pubmed.ncbi.nlm.nih.gov/27318475 27318475]|[https://pubmed.ncbi.nlm.nih.gov/31346146 31346146]|[https://pubmed.ncbi.nlm.nih.gov/7648586 7648586]|[https://pubmed.ncbi.nlm.nih.gov/24236059 24236059]|[https://pubmed.ncbi.nlm.nih.gov/21964054 21964054]|[https://pubmed.ncbi.nlm.nih.gov/27456773 27456773]|[https://pubmed.ncbi.nlm.nih.gov/30558911 30558911]|[https://pubmed.ncbi.nlm.nih.gov/21559451 21559451]|[https://pubmed.ncbi.nlm.nih.gov/29312455 29312455]|[https://pubmed.ncbi.nlm.nih.gov/27549315 27549315]|[https://pubmed.ncbi.nlm.nih.gov/30599359 30599359]|[https://pubmed.ncbi.nlm.nih.gov/6141938 6141938]|[https://pubmed.ncbi.nlm.nih.gov/28883415 28883415]|[https://pubmed.ncbi.nlm.nih.gov/30733757 30733757]|[https://pubmed.ncbi.nlm.nih.gov/26041528 26041528]|[https://pubmed.ncbi.nlm.nih.gov/16643871 16643871] <br> | ||
'''definition''' : An amino trisaccharide consisting of β-D-galactopyranose, 2-acetamido-2-deoxy-β-D-galactopyranose and α-D-galactopyranose residues joined in sequence by (1→3) glycosidic linkages.[CHEBI:149071] <br> | '''definition''' : An amino trisaccharide consisting of β-D-galactopyranose, 2-acetamido-2-deoxy-β-D-galactopyranose and α-D-galactopyranose residues joined in sequence by (1→3) glycosidic linkages.[CHEBI:149071] <br> | ||
'''term_xref''' : GTC:G25129BT|GlycoEpitope:EP0039|CID: | '''term_xref''' : GTC:G25129BT|GlycoEpitope:EP0039|CID:91855781|CHEBI:149071|GlycoMotif:GGM.000079 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
| Line 647: | Line 647: | ||
'''glycan_dictionary_accession''' : GSD000161 <br> | '''glycan_dictionary_accession''' : GSD000161 <br> | ||
'''glytoucan_accession ''' : [https://www.glygen.org/glycan/G84263XI G84263XI] <br> | '''glytoucan_accession ''' : [https://www.glygen.org/glycan/G84263XI G84263XI] <br> | ||
'''term_in_sentence''' : Glycosphingolipids (GSLs), such as the globo-series GSLs stage-specific embryonic antigen 3 (SSEA-3), SSEA-4, and Globo-H, are specifically expressed on pluripotent stem cells and cancer cells, and are known to be associated with various biological processes such as cell recognition, cell adhesion, and signal transduction.[PMID: | '''term_in_sentence''' : Glycosphingolipids (GSLs), such as the globo-series GSLs stage-specific embryonic antigen 3 (SSEA-3), SSEA-4, and Globo-H, are specifically expressed on pluripotent stem cells and cancer cells, and are known to be associated with various biological processes such as cell recognition, cell adhesion, and signal transduction.[PMID:[https://pubmed.ncbi.nlm.nih.gov/31817926 31817926]] <br> | ||
'''publication''' : [https://pubmed.ncbi.nlm.nih.gov/27845370 27845370]| | '''publication''' : [https://pubmed.ncbi.nlm.nih.gov/27845370 27845370]|[https://pubmed.ncbi.nlm.nih.gov/31817926 31817926]|[https://pubmed.ncbi.nlm.nih.gov/26295543 26295543]| [https://pubmed.ncbi.nlm.nih.gov/25171543 25171543]|[https://pubmed.ncbi.nlm.nih.gov/25978997 25978997]|[https://pubmed.ncbi.nlm.nih.gov/30296654 30296654]|[https://pubmed.ncbi.nlm.nih.gov/25853231 25853231]|[https://pubmed.ncbi.nlm.nih.gov/24862940 24862940]|[https://pubmed.ncbi.nlm.nih.gov/31243630 31243630]|[https://pubmed.ncbi.nlm.nih.gov/28736232 28736232]|[https://pubmed.ncbi.nlm.nih.gov/22895512 22895512]|[https://pubmed.ncbi.nlm.nih.gov/24578244 24578244]|[https://pubmed.ncbi.nlm.nih.gov/29478902 29478902]|[https://pubmed.ncbi.nlm.nih.gov/23574147 23574147]|[https://pubmed.ncbi.nlm.nih.gov/23768762 23768762]|[https://pubmed.ncbi.nlm.nih.gov/27797400 27797400]|[https://pubmed.ncbi.nlm.nih.gov/29227830 29227830]| [https://pubmed.ncbi.nlm.nih.gov/18773292 18773292]|[https://pubmed.ncbi.nlm.nih.gov/25123923 25123923]|[https://pubmed.ncbi.nlm.nih.gov/22924692 22924692]|[https://pubmed.ncbi.nlm.nih.gov/22665977 22665977]|[https://pubmed.ncbi.nlm.nih.gov/23330736 23330736]|[https://pubmed.ncbi.nlm.nih.gov/31831622 31831622]|[https://pubmed.ncbi.nlm.nih.gov/31916611 31916611]| [https://pubmed.ncbi.nlm.nih.gov/2412924 2412924]|[https://pubmed.ncbi.nlm.nih.gov/16033656 16033656]|[https://pubmed.ncbi.nlm.nih.gov/24866102 24866102]| [https://pubmed.ncbi.nlm.nih.gov/20458727 20458727]|[https://pubmed.ncbi.nlm.nih.gov/[https://pubmed.ncbi.nlm.nih.gov/21149348 21149348] [https://pubmed.ncbi.nlm.nih.gov/21149348 21149348]]|[https://pubmed.ncbi.nlm.nih.gov/26940129 26940129]| [https://pubmed.ncbi.nlm.nih.gov/17008424 17008424]| [https://pubmed.ncbi.nlm.nih.gov/2874662 2874662]|[https://pubmed.ncbi.nlm.nih.gov/23301556 23301556]|[https://pubmed.ncbi.nlm.nih.gov/27836003 27836003]|[https://pubmed.ncbi.nlm.nih.gov/19118716 19118716]|[https://pubmed.ncbi.nlm.nih.gov/24550271 24550271]|[https://pubmed.ncbi.nlm.nih.gov/32988880 32988880]|[https://pubmed.ncbi.nlm.nih.gov/22743677 22743677]|[https://pubmed.ncbi.nlm.nih.gov/20683402 20683402]|[https://pubmed.ncbi.nlm.nih.gov/17062733 17062733]|[https://pubmed.ncbi.nlm.nih.gov/23570331 23570331]|[https://pubmed.ncbi.nlm.nih.gov/30310530 30310530]|[https://pubmed.ncbi.nlm.nih.gov/31545316 31545316]|[https://pubmed.ncbi.nlm.nih.gov/23509763 23509763]|[https://pubmed.ncbi.nlm.nih.gov/24364909 24364909]|[https://pubmed.ncbi.nlm.nih.gov/18273440 18273440]|[https://pubmed.ncbi.nlm.nih.gov/28985528 28985528]|[https://pubmed.ncbi.nlm.nih.gov/22266579 22266579] <br> | ||
'''definition''' : <br> | '''definition''' : <br> | ||
'''term_xref''' : GTC:G84263XI|GlycoEpitope:EP0044|CID: | '''term_xref''' : GTC:G84263XI|GlycoEpitope:EP0044|CID: 91845478|CHEBI:154843 <br> | ||
'''synonyms''' : <br> | '''synonyms''' : <br> | ||
'''function''' : <br> | '''function''' : <br> | ||
Revision as of 23:32, 9 April 2023
Monosialylated
term (main_entry) : Monosialylated
glycan_dictionary_accession : GSD000123
glytoucan_accession :
term_in_sentence : The structures of the asparagine-linked sugar chains of U- CD59 were biantennary complex type , only 4.2% of which are monosialylated.[PMID:7514386]
publication : 7514386|8477709|8942648|29752599|31669586|29747818|16425369|12089170|31390883|1632647|29733234|31110965|31566965|30555678|28263871|29652662|25915761|16381065|29247593|28370937|23896595|26633899|32155999|25261856|2737211|26945091|27065039|26784838|26582205
definition : A glycan that have the addition of one sialic acid residues.
term_xref :
synonyms :
function : appears in bovine brain gangliosides.[PMID: 29752599]
disease_associations :
wikipedia :
essentials_of_glycobiology :
Monosialyl-biantennary
term (main_entry) : Monosialyl-biantennary
glycan_dictionary_accession : GSD000124
glytoucan_accession :
term_in_sentence : In contrast, the sugar chain structure of HCC highly specific AFP isoform was found to comprise a monosialyl-biantennary sugar chain with additional fucosylation of the proximal N-acetylglucosamine.[PMID:7686446]
publication : 7686446|1374031|9648261|10460833|19524219|10207016|15797573|15218190
definition : A biantennary glycan with two GlcNAc branches linked to the core and the addition of one sialic acid residues.
term_xref :
synonyms : monosialyl biantennary|sialylated diantennary|sialylated biantennary
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
Monosialyl-Gb5
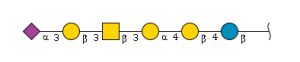
term (main_entry) : Monosialyl-Gb5
glycan_dictionary_accession : GSD000125
glytoucan_accession : G32254QI
term_in_sentence : Thus, malignancy of MCF-7 is highly dependent on monosialyl-Gb5, and its activation of cSrc and FAK in GEM.[PMID:12401210]
publication : 12401210|16995838|27318475
definition : alpha-Neup5Ac-(2->3)-beta-D-Galp-(1->3)-beta-D-GalpNAc-(1->3)-alpha-D-Galp-(1->4)-beta-D-Galp-(1->4)-beta-D-Glcp is a linear amino hexasaccharide comprising β-D-glucose at the reducing end with at the 4-position a β-D-galactosyl-(1→3)-N-acetyl-β-D-glucosaminyl-(1→3)-α-D-galactosyl-(1→4)-β-D-galactosyl moiety, α(2→3)-sialylated at the terminal galactosyl residue.[CHEBI:62313]
term_xref : GTC:G32254QI|GlycoEpitope:EP0099|GlycoMotif:GGM.000080|CID:73427363|CHEBI:62313
synonyms : GL-7 globoseries ganglioside (SSEA-4)|Monosialyl Gb5|Sialosyl galactosyl globoside|GL-7 globoseries ganglioside|SSEA-4
function :
disease_associations : renal cell carcinoma/RCC[GlycoEpitope:EP0099]
wikipedia :
essentials_of_glycobiology :
Mono-sulfated globopentaosylceramide
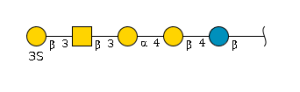
term (main_entry) : Mono-sulfated globopentaosylceramide
glycan_dictionary_accession : GSD000126
glytoucan_accession : G80193TN
term_in_sentence : Mono-sulfated globopentaosylceramide from human kidney.[PMID:2777788]
publication : 2777788
definition :
term_xref : GlycoMotif:GGM.000072|GTC:G80193TN
synonyms : Monosulfated globopentaosylceramide
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
Mono-sulfated globotetraosylceramide
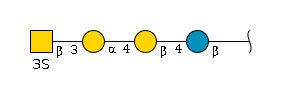
term (main_entry) : Mono-sulfated globotetraosylceramide
glycan_dictionary_accession : GSD000127
glytoucan_accession : G05297EK
term_in_sentence : Mono-sulfated globotetraosylceramide from human kidney.[PMID:2559078]
publication : 2559078
definition :
term_xref : GlycoMotif:GGM.000073|GTC:G05297EK
synonyms :
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
N-acetyl GM2
term (main_entry) : N-acetyl GM2
glycan_dictionary_accession : GSD000128
glytoucan_accession : G79389NT
term_in_sentence : The appearance of the N-acetyl GM2 antigen correlated well with the degree of differentiation of the cancer cells in patients with squamous cell carcinoma and adenocarcinoma of the lung.[PMID:3167861]
publication :3167861|2153431| 25673107
definition : beta-D-GalpNAc-(1->4)-[alpha-Neup5Ac-(2->3)]-beta-D-Galp-(1->4)-beta-D-Glcp is a branched amino tetrasaccharide consisting of the linear sequence β-D-GalNAc-(1→4)-β-D-Gal-(1→4)-β-D-Glc having a Neu5Ac residue attached to the galactose via an α-(2→3) linkage. Corresponds to the carbohydrate portion of ganglioside GM2.[CHEBI:59220]
term_xref : GTC:G79389NT|GlycoEpitope:EP0051|CID: 45266845|CHEBI:59220
synonyms :
function : The biological role played by a material entity when bound by a receptor of the adaptive immune system. Specific site on an antigen to which an antibody binds.[CHEBI:59220]
disease_associations : lung adenocarcinoma[GlycoEpitope:EP0051]|embryonal carcinoma[GlycoEpitope:EP0051]|germ cell tumor[GlycoEpitope:EP0051]|teratocarcinoma[GlycoEpitope:EP0051]|squamous cell carcinoma[GlycoEpitope:EP0051]
wikipedia :
essentials_of_glycobiology :
N-acetyllactosamine (Type 1)
term (main_entry) : N-acetyllactosamine (Type 1)
glycan_dictionary_accession : GSD000181
glytoucan_accession : G00056MO
term_in_sentence : The N-acetyllactosamine type 1 (LacNAc type 1, Galβ1,3GlcNAc) is a well-known precursor of several important blood group epitopes, such as Lewis A, Lewis B, or sialyl Lewis A [1], which are involved in many biological processes, e.g., fertilization [2] and pathogen adhesion [3]. [PMID:28796164]
publication : 28796164|28167527
definition :
term_xref : GTC:G00056MO|GlycoMotif:GGM.000005
synonyms : LacNAc type 1|Type 1 LN|Type-1 lactosamine
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
N-acetyllactosamine (Type 2)
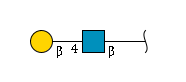
term (main_entry) : N-acetyllactosamine (Type 2)
glycan_dictionary_accession : GSD000129
glytoucan_accession : G00055MO
term_in_sentence : Utilising a fast and sensitive screening method based on imidazolium-tagged probes, we report unprecedented reversible activity of bacterial beta1,4-galactosyltransferases to catalyse the transgalactosylation from lactose to N-acetylglucosamine to form N-acetyllactosamine in the presence of UDP.[PMID:31165848]
publication : 31165848|31126011|28796164|6236210|9751793|29740087|9151975|10567443|21472942|26477523|6548353|28167527|20056550|7737204|22052010|10223649|11323440|10420596|7766648|25257940|17977857|15556936|26541999|23650594|9692202|29429899|21062783|25034434|8985145|28503789|8720464/
definition : N-acetyllactosamine is a beta-D-galactopyranosyl-(1→4)-N-acetyl-D-glucosamine having beta-configuration at the reducing end anomeric centre. It is also known as galb1-4glcnacb or lacnac, belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. N-Acetyllactosamine is an extremely weak basic (essentially neutral) compound (based on its pKa). N-Acetyllactosamine exists in all living organisms, ranging from bacteria to humans.[CHEBI:16153,HMDB0001542]
term_xref : GTC:G00055MO|CID:439271|CHEBI:16153|MeSH:C000458|GlycoMotif:GGM.000001
synonyms : LacNAc|Type 2 LN
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
N-Glycolyl-GM2
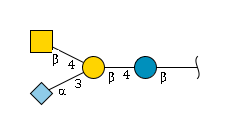
term (main_entry) : N-Glycolyl-GM2
glycan_dictionary_accession : GSD000130
glytoucan_accession : G56478LD
term_in_sentence : The monoclonal antibodies MK2-34 and MK1-16 (both IgM), which specifically detect N-glycolyl GM2 and N-acetyl GM2, respectively, were generated by immunizing mice with liposomes containing monophosphoryl lipid A, trehalose dimycolate, and the antigenic ganglioside.[PMID:3167861]
publication : [3167861|11861662|17980710|3731079| 25673107]
definition : (2S,4S,5R,6R)-2-[(2R,3S,4R,5R,6S)-3-[(2S,3R,4R,5R,6R)-3-Acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-5-hydroxy-2-(hydroxymethyl)-6-[(2R,3S,4R,5R,6R)-4,5,6-trihydroxy-2-(hydroxymethyl)oxan-3-yl]oxyoxan-4-yl]oxy-4-hydroxy-5-[(2-hydroxyacetyl)amino]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid is a member of carbohydrates and carbohydrate derivatives.[CHEBI:151464]
term_xref : GTC:G56478LD |GlycoEpitope:EP0052|CID:91845742|CHEBI:151464|GlycoEpitope:GGM.000096
synonyms :
function :
disease_associations : choriocarcinoma[GlycoEpitope:EP0052]|teratocarcinoma|[GlycoEpitope:EP0052]yolk sac tumor[GlycoEpitope:EP0052]
wikipedia :
essentials_of_glycobiology :
N-linked glycans
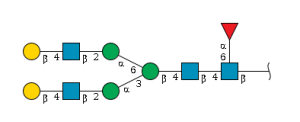
term (main_entry) : N-linked glycans
glycan_dictionary_accession : GSD000131
glytoucan_accession : G78059CC
term_in_sentence : Our study identifies extracellular N-linked glycans-and the glycoprotein neurexin 1β specifically-as key modulators of neuronal uptake of αSacetyl, drawing attention to the potential therapeutic value of αSacetyl-glycan interactions.[PMID:31211781]
publication : 31211781|28384382|30305477|31364612|26899268|29945790|29652253|29723274|30962676|29778448|25325701|31100702|28898525|22944671|19277537|24473128|17957771|27554083|15189166|11269317|19577919|24303005|12042249|22191536|28324664|25965797|25434632|30484244|28110657| 29865036|20129637|27346875|30280442|26911286|22491358|26582281|26398792|31548313|31066275|28331984|25482090| 28518173|30733536|22326428|30063825|30315103|21558494|31676555|31256374
definition : The carbohydrate portion of a glycoprotein that has a glycan linked through the nitrogen of an asparagine side-chain.[CHEBI:59520]
term_xref : GTC:G78059CC|CID: 70679232|CHEBI:70967
synonyms :
function : N-glycans modulate the function of several cell surface proteins which involved in migration, adhesion and which are responsible for regulating myelination.[PMID: 25151374]|Affect transportation of the glycosylated proteins in the Golgi.[PMID: 25151374]|Affects cell-cell interaction, adhesion, expression, proper folding.[PMID: 25151374]|extracellular N-glycans act as key modulators of neuronal uptake of αacetyl [PMID: 31211781].
disease_associations : Rheumatoid arthritis[PMID:17392038]|Type 1 Diabetes[PMID:29146600]|Lung Cancer[PMID: 23766031]
wikipedia :
essentials_of_glycobiology : Chapter 14
Nonfucosylated
term (main_entry) : Nonfucosylated
glycan_dictionary_accession : GSD000132
glytoucan_accession :
term_in_sentence : In the carbohydrate moiety , alpha-subunit from normal pregnancy hCG contained nonfucosylated , mono- and biantennary N-linked structures (49.3 and 36.7%, means); fucosylated biantennary and triantennary oligosaccharides were also identified (7.3 and 6.9%).[PMID:9449027]
publication : 9449027 |30258536|23554380|16675584|26747427|22457527|24362443|19657637|8811881|19218011|20022111|17363544|17368483|20564614|27054024|28397880|31490688|31860772|17012310|29633346|9442021|30350565|30296068|31800250|30021910|31133022| 29224385|[https://pubmed.ncbi.nlm.nih.gov/28630087 28630087 28630087]|26444434|31697975|30991260
definition : glycans without a fucose component/monosaccharide residue.
term_xref :
synonyms :
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
Nonfucosylated biantennary
term (main_entry) : Nonfucosylated biantennary
glycan_dictionary_accession : GSD000133
glytoucan_accession :
term_in_sentence : Structural analysis combining methylation-mass spectrometry and 400 MHz 1H-n.m.r. spectrometry of oligosaccharide alditols released from human leucocyte lactotransferrin shows the presence of two disialylated and non-fucosylated biantennary glycans of the N-acetyl-lactosaminic type.[PMID: 2390069]
publication : 2390069| 9449027 |1375510|9101715|2332109
definition : A biantennary glycan with two GlcNAc branches linked to the core and no fucose appears.
term_xref :
synonyms :
function :
disease_associations :
wikipedia :
essentials_of_glycobiology : Chapter 9
Nonfucosylated biantennary N-Linked structures
term (main_entry) : Nonfucosylated biantennary N-Linked structures
glycan_dictionary_accession : GSD000134
glytoucan_accession :
term_in_sentence : The beta-subunit from normal pregnancy hCG contained fucosylated and nonfucosylated biantennary N-linked structures; however, mono- and triantennary oligosaccharides were also identified (4.6 and 13.7%).[PMID:9449027]
publication : 9449027
definition : A biantennary N-linked glycan with two GlcNAc branches linked to the core and no fucose appears.
term_xref :
synonyms :
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
Nonfucosylated triantennary
term (main_entry) : Nonfucosylated triantennary
glycan_dictionary_accession : GSD000135
glytoucan_accession :
term_in_sentence : Carbohydrate structures of human alpha-fetoprotein of patients with hepatocellular carcinoma: presence of fucosylated and non-fucosylated triantennary glycans.[PMID:7679920]
publication : 7679920|23274891
definition : A triantennary N-linked glycan with three GlcNAc branches linked to the core and no fucose appears.
term_xref :
synonyms :
function : Appears in human alpha-fetoprotein (AFP).[PMID:7679920]
disease_associations : hepatocellular carcinoma[PMID:7679920].
wikipedia :
essentials_of_glycobiology :
Nonsialylated
term (main_entry) : Nonsialylated
glycan_dictionary_accession : GSD000136
glytoucan_accession :
term_in_sentence : The pattern of nonsialylated oligosaccharides was used for interpretation of the fully sialylated species using bioinformatics tools. From pooled human plasma, we find 90, 101, and 64 different glycan compositions for genetic variants ORM1*F1, ORM1*S, and ORM2, respectively. Glycan structures carry dominantly between 15 and 16 sialic acids indicating an almost complete termination of all antenae with sialic acid.[PMID:30295034]
publication : 14533820|1377689|9705949|30788437|28090561|12498371|26839900| 31490688|2656426|30295034|31545048|12381155|25730103|26787879|25646460| 29224385|26063435|26954852|32889432|2825412|27504786|22431161
definition : A glycan which is not sialylated.
term_xref :
synonyms :
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
O-fucose glycans
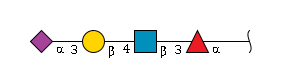
term (main_entry) : O-fucose glycans
glycan_dictionary_accession : GSD000137
glytoucan_accession : G39294YF
term_in_sentence : Recently, a number of laboratories have shown that O-fucose glycans on the epidermal growth factor (EGF)-like repeats of the Notch extracellular domain modulate Notch signaling.[PMID:12417415]
publication : 19594638|30690220| 18952191|22415200|12417415|16400812|18948267|21464368|20816394|30207383|20301232|27129198|17132502|22492969|30030822|30214079|26175457|25378397|18272537|28729422|11524432|31722217|23045360|24803430|28876865|25700513|32518939|30523691|18227520|20816217|24909690|17964136|12036964|27268051|15653671|19948734|31590629|32201074|15189151|17263732|32913123|12909620|28785176|17132500|22949680|25053492|28939751
definition : Form of glycosylation where the protein is modified by a fucose residue and can be extended futher to form a tetrasaccharide in certain cases. alpha-linked o-fucose have a consensus motif of C2X4(s/T)C3 where C2 and C3 are conserved cysteines number 2 and 3 respectively of the EGFR like repeats. [Essentials of Glycobiology:Chapter13]
term_xref : GTC:G39294YF|GlycoEpitope:EP0005
synonyms :
function : modification of Notch signaling.[GlycoEpitope:EP0005]
disease_associations :
wikipedia :
essentials_of_glycobiology :
O-GlcNAc
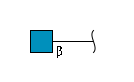
term (main_entry) : O-GlcNAc
glycan_dictionary_accession : GSD000138
glytoucan_accession : G49108TO
term_in_sentence : A better understanding of the mechanisms of OGT/OGA action would enable us to derive therapeutic benefits of resetting cellular O-GlcNAc levels within an optimal range.[PMID:30464755]
publication : 30464755|29049853|29594839|31654859|29404877|30105004|30669087|29790000|28408483|26862193|30523150|30037904|28408480|29352075|29456783|29577901|29223644|28638491|30221662|29904918|32155042|30298013|31630803|31847126|29784830|30181664|28768194|30134155|25336654|30356792|30199580|32329777|28408494|29774032|29044951|23836420|20301273|29772801|28922739|30100348|30793403|30657688|31237748|29756380|29954943|30018219|32207184|25566193|30626734|24759912|
definition : Form of glycosylation which exclusively occurs in the mitochondrial, cytoplasmic or nuclear compartments of the cell where the protein is modified by a GlcNAc monosaccharide on the serine or threonine residues and is generally not modified or elongated further to form more complex structures. [Essentials of Glycobiology:Chapter19]
term_xref : GTC:G49108TO|GlycoEpitope:EP0004|CID:24139|HMDB:HMDB0000803
synonyms :
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
O-linked glycans
term (main_entry) : O-linked glycans
glycan_dictionary_accession : GSD000139
glytoucan_accession :
term_in_sentence : Among these, six fucosylated N-linked glycansand four O-linked glycans exhibited significantly increased expression levels in GC, while five fucosylated N-linked glycans and ten fucosylated O-linked glycans exhibited significantly decreased expression levels in GC.[PMID:29865036]
publication : 29865036|30952424|31611643|19577919|32019882|19139197|26184710|11159917|21536259|25186198|19277556|22261557|24115046|18725413|15966855|24256304|25477510|10359703|24406064|22259135|29279989| 15966855|30619255|9673446| 31676555|28745859|28196325|27975143|30591584|[https://pubmed.ncbi.nlm.nih.gov/28518173 28518173 28518173]|17522109| 26328495|32178593|27529638|24663386|22474328|28096352|27496768| 18952191|11135308|24917611|11532966|22786570|11891229|18332077|26867212|26925665|16769205|23475718|16005634|28785176
definition : The carbohydrate portion of a glycoprotein that has a glycan linked through the hydroxyl oxygen of serine, threonine, hydroxylysine or hydroxyproline side-chains.[CHEBI:59521]
term_xref : CHEBI:59521
synonyms :
function : Affects several important activities like protein stability, modulation of signaling molecules and enzyme activity.[PMID:9673446]
disease_associations :
wikipedia :
essentials_of_glycobiology :
O-linked mannose
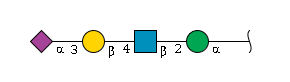
term (main_entry) : O-linked mannose
glycan_dictionary_accession : GSD000140
glytoucan_accession : G59126YU
term_in_sentence : Protein O-linked mannose beta-1,4-N-acetylglucosaminyltransferase 2 (POMGNT2) catalyzes the first step toward the functional matriglycan structure on alpha-dystroglycan that is responsible for binding extracellular matrix proteins and certain arenaviruses.[PMID: 27932460]
publication : 27932460|24526361|25381909|26644575|23851827|28810660|19054127|23929950|21684258|22608994|28973932|24733390|31851597|29884773|28711406|14577328|21089343|14617637|9838223|17072003| 26328495|11181560|31949166|31988979|20362685|27733679|27493216|20044576|30643095|29732660|10580142|14568618|28079948|15467391|27812179|23761899|25737452|32930586|25381372|23434682|16857188|21452199|19429925|25361541|24297939|30322079|23428289|28427937|22746206|27533452|17502374|20816174|27496765
definition : O-linked glycans that are initiated by the addtion of a Man residue to Ser or Thr. Subsequent extension of the Man defines the O-Man core type, either M1, M2, or M3. The M1 type is initated by addition of a GlcNAc in β-linkage to the 2 position of the Man residue. M2 is initiated by addition of another GlcNAc to the 6 position of the M1 core. M3 is initiated by the addition of a GlcNAc in β-linkage to the 4 position of the Man residue. M1 and M2 type core glycans can be extended and branched with Gal, Fuc, Sialic Acid, and GlcA. The M3 core is uniquely extended by a multienzyme system to generate the matriglycan polysaccharide, a protein-specific modification found only on alpha-dystroglycan. [CHEBI:87761]
term_xref : GlycoMotif:GGM.000055|GTC:G59126YU|CHEBI:87761|GlycoEpitope:EP0002|CID: 91859038
synonyms : O-mannosyl glycan
function :
disease_associations : MEB/muscle-eye-brain disease[GlycoEpitope:EP0002]|WWS/walker-warburg syndrome |muscular dystrophy[GlycoEpitope:EP0002]|neuronal migration disorder[GlycoEpitope:EP0002]|Muscle-Eye-Brain disease (MEB) and Walker-Warburg Syndrome (WWS) have mutations in genes encoding glycosyltransferases needed for O -mannosyl oligosaccharide synthesis[GlycoEpitope:EP0002]|Muscular dystrophy and neuronal migration disorder are caused by mutations in a glycosyltransferase, POMGnT1.[GlycoEpitope:EP0002]
wikipedia :
essentials_of_glycobiology :
P1 antigen
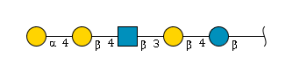
term (main_entry) : P1 antigen
glycan_dictionary_accession : GSD000141
glytoucan_accession : G12460DL
term_in_sentence : The P1 antigen was disovered in 1927 by Landsteiner and Levine, and Pk and NOR were described in 1951 and 1982, respectively.[PMID: 24046920]
publication : 29622537|26773500|24046920|6207232|8550516|6609976|6375319|817855|2428855|3312011|15364312|1937801|7729906
definition : An amino pentasaccharide consisting of α-D-galactose, β-D-galactose N-acetyl-α-D-glucosamine, β-D-galactose, and β-D-glucose residues joined in sequence with ((1→4)-, (1→4)-, (1→3)- and (1→4)-linkages, respectively.[CHEBI:68484]
term_xref : GlycoMotif:GGM.000069|GTC:G12460DL|CID:71296137|CHEBI:68484|SugarBind_Ligand:160
synonyms :
function :
disease_associations : Shigellosis[SugarBind_Ligand:160]
wikipedia :
essentials_of_glycobiology : Chapter 23
Para-Forssman glycolipid
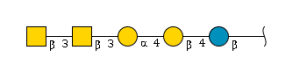
term (main_entry) : Para-Forssman glycolipid
glycan_dictionary_accession : GSD000142
glytoucan_accession : G51699SI
term_in_sentence : A novel pentaglycosyl ceramide containing di-beta-N-acetylgalactos-aminyl residue (Para-Forssman glycolipid) isolated from human erythrocyte membrane. [PMID:6814203]
publication : 6814203
definition : GalNAcβ1→3GalNAcβ1→3Galα1→4Galβ1→4Glcβ1→1Cer [https://doi.org/10.1016/S0040-4039(01)93814-6]
term_xref : GlycoMotif:GGM.000085|GTC:G51699SI|CID:56836111|KEGG:G00096|SugarBind_Ligand:19|CHEBI:151565
synonyms :
function :
disease_associations : Actinomycosis[SugarBind_Ligand:19]
wikipedia : https://en.wikipedia.org/wiki/P1PK_blood_group_system
essentials_of_glycobiology :
Paragloboside
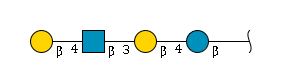
term (main_entry) : Paragloboside
glycan_dictionary_accession : GSD000143
glytoucan_accession : G58896AZ
term_in_sentence : Galactosyltransferase activities in sera of cancer patients were determined by assaying the formation of paragloboside from UDP-galactose and lactotriaosylceramide immobilized on microtiter plates by means of the enzyme-linked immunosorbent assay using a monoclonal antibody, H-11, directed to paragloboside. [PMID: 1551117]
publication : 3345524|850070|10569746|30624951|29438961|6816790|1551117|6865961|25039255
definition : α-Neup5Ac-(2→3)-β-D-Galp-(1→3)-D-GalpNAc in which the configuration at the GalNAc anomeric carbon is α.[CHEBI:65257]
term_xref : GlycoMotif:GGM.000077|GTC:G58896AZ|CID: 21670519|CHEBI:65257|GlycoEpitope:EP0075|SugarBind_Ligand:15
synonyms :
function :
disease_associations : Pharyngitis[SugarBind_Ligand:15]|Tracheobronchitis[SugarBind_Ligand:15]|Gonorrhea[SugarBind_Ligand:15]|Actinomycosis[SugarBind_Ligand:15]|Diarrhea[SugarBind_Ligand:15]|Hemorrhagic colitis[SugarBind_Ligand:15]|Chronic gastritis[SugarBind_Ligand:15]|Peptic ulcers[SugarBind_Ligand:15]|Gastric cancer[SugarBind_Ligand:15]|Influenza[SugarBind_Ligand:15]
wikipedia :
essentials_of_glycobiology : Chapter 14
Paucimannose
term (main_entry) : Paucimannose
glycan_dictionary_accession : GSD000144
glytoucan_accession :
term_in_sentence : The galactosylation of core fucose (GalFuc epitope) in paucimannose and complex-type N-glycans is characteristic of protostome organisms, including flatworms (planarians).[PMID:29475940]
publication : 29475940|29803109|12603202|31817246|31719144|21057214|31932305|17132514|30382146|27731363|31325506|25746926|26513758|16522637|21169367|25190359|27928741|22496646|27384337|16897177|15228383|27379103|27189951|23475714|24907509|20558211|28060516|20441997|27720757|28817611|24550396|19494052|19515361|28630087|31320997|30651366|30253927|25922361|31655162|18056652|24467287|32367383
definition : Any N-linked glycan derivative with composition Man1-4Fuc0-1 GlcNAc2, in which the chitobiose core (GlcNAc2) is intact. Found in relatively large amounts in invertebrates and plants, they are formed by removal of the non-reducing terminal GlcNAc (\added by GlcNAcT1) from GlcNAcMan3-5GlcNAc2 by a Golgi hexosaminidase and subsequent removal of Man residues by Golgi mannosidases. Pauscimannose glycans have also been detected in vertebrates.[CHEBI:144386]
term_xref : CHEBI:144386
synonyms :
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
Polysialic acid
term (main_entry) : Polysialic acid
glycan_dictionary_accession : GSD000182
glytoucan_accession :
term_in_sentence : Polysialic acid (PSA) is a carbohydrate composed of a linear polymer of α2,8-linked sialic acids, primarily attached to the Neural Cell Adhesion Molecule (NCAM). [PMID:36076963]
publication : 36076963|35222358
definition :
term_xref : GlycoMotif:GGM.000252
synonyms : polySia|PSA
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
Sd(A)/Cad
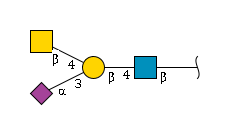
term (main_entry) : Sd(A)/Cad
glycan_dictionary_accession : GSD000145
glytoucan_accession : G41052GC
term_in_sentence : The expanding roles of the Sd(a)/Cad carbohydrate antigen and its cognate glycosyltransferase B4GALNT2.[PMID:24112972]
publication : 24112972|11577689|30067891|15361072
definition : A branched amino tetrasaccharide comprised of a trisaccharide chain of N-acetyl-α-neuraminic acid, β-D-galactose and N-acetyl-β-D-glucosamine residues linked sequentially (2→3) and (1→4), to the galactose residue of which is also linked (1→4) an N-acetyl-β-L-galactosamine residue. [CHEBI:71561]
term_xref : GlycoMotif:GGM.000051|GTC:G41052GC|CID:70698375|CHEBI:71561
synonyms : Sd^a /Cad
function :
disease_associations :
wikipedia :
essentials_of_glycobiology : Chapter 9
SDlex
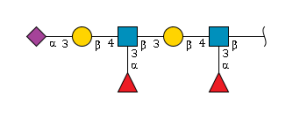
term (main_entry) : SDlex
glycan_dictionary_accession : GSD000146
glytoucan_accession : G19619KS
term_in_sentence : Hepatic expression of sialylated difucosyl Lex antigen (SDLex, NeuAc alpha 2-3Gal beta 1-4(Fuc alpha 1-3)GlcNAc beta 1-3Gal beta 1-4(Fuc alpha 1-3)GlcNAc beta 1-) was studied with monoclonal antibody FH6, which defines this structure.[PMID: 3341453]
publication : 30226570|3341453|12725333
definition : (2S,4S,5R,6R)-5-Acetamido-2-[(2S,3R,4S,5S,6R)-2-[(2R,3S,4R,5R,6S)-5-acetamido-6-[(2S,3R,4S,5S,6R)-2-[(2R,3S,4R,5R,6R)-5-acetamido-6-hydroxy-2-(hydroxymethyl)-4-[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-3-yl]oxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-(hydroxymethyl)-4-[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-3-yl]oxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-4-hydroxy-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid is a member of neuraminic acids.[CHEBI:148203]
term_xref : GlycoMotif:GGM.000032|GTC:G19619KS|CID:91856705|CHEBI:148203
synonyms : Sdlex|SDLe^x|SDLex
function :
disease_associations :
wikipedia :
essentials_of_glycobiology : Chapter 14
Seminolipid
term (main_entry) : Seminolipid
glycan_dictionary_accession : GSD000147
glytoucan_accession : G72548RZ
term_in_sentence : A concise total synthesis of seminolipid, a sulfoglycolipid, has been achieved; key features include regioselective, tin-free sulfation of allyl β-d-galactopyranoside using 2,6-bis(trifluoromethyl)phenylboronic acid as protective reagent, stereoselective epoxidation, and site-selective acylation. [PMID: 31353379]
publication : 31353379|31736776|12810822|19582571|19542524|20670037|15659616|20817833|870059|10801776|21965315|8095813|1280685|4690236|34199863|4207869|2373957|845131
definition :
term_xref : GlycoMotif:GGM.000062|GTC:G72548RZ
synonyms :
function :
disease_associations :
wikipedia :
essentials_of_glycobiology : Chapter 14
Sialopentaosylceramide
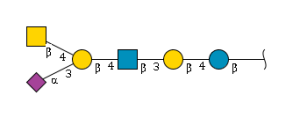
term (main_entry) : Sialopentaosylceramide
glycan_dictionary_accession : GSD000148
glytoucan_accession : G13224CR
term_in_sentence : Sialylpentaosylceramide detected with anti-GM2 monoclonal antibody. Structural characterization and complementary expression with GM2 in gastric cancer and normal gastric mucosap[PMID: 2139874]
publication : 2139874
definition : A ganglioside in which the oligosaccharide portion is composed of a pentasaccharide containing one or more sialic acid residues.[CHEBI:36542]
term_xref : GlycoMotif:GGM.000097|GTC:G13224CR|CID: 91859576|CHEBI:148045|KEGG:G00883|CHEBI:36542
synonyms :
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
Sialosyl paragloboside
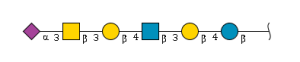
term (main_entry) : Sialosyl paragloboside
glycan_dictionary_accession : GSD000149
glytoucan_accession : G50123ZO
term_in_sentence : Unlike CNS myelin, human peripheral nerve myelin has the acidic glycosphingolipids sialosyl paragloboside (SPG), sialosyl lactosaminyl paragloboside (SLPG), and sulfated glucuronyl paragloboside (SGPG).[PMID:8862128]
publication : 8862128|7236652|2370108|3133978|2905604|1749114|1573418
definition : α-Neup5Ac-(2→3)-β-D-Galp-(1→4)-D-GlcpNAc with β configuration at the anomeric position of the reducing-end N-acetyl-D-glucosamine residue.[CHEBI:71612]
term_xref : GlycoMotif:GGM.000081|GTC:G50123ZO|CID:91845504|KEGG:G01429|CHEBI:151914
synonyms :
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
Sialyl Lewis a
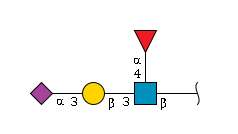
term (main_entry) : Sialyl Lewis a
glycan_dictionary_accession : GSD000150
glytoucan_accession : G00053MO
term_in_sentence : By comparing the serum O-glycan profiles from healthy controls with those of cancer patients, we identified a marker candidate, core 1 sialyl Lewis A (NeuAcα2-3Galβ1-3(Fucα1-4)GlcNAcβ1-3Gal) (abbreviated C1SLA), whose concentration appeared to be weakly correlated with CA19-9 values.[PMID:26641888]
publication : 26641888|31424423|26530370|17760270|18343410|16033091|8829184|11907351|20592391|26641888|24128856|30249386|10773785|16149606|10996727|10436809|10667238|7720892|7630017|10612415|9836451|12511721|22623153
definition : A sialyated version of Lewis A with a branched structure of amino tetrasaccharide consisting of a sialyl residue, linked (2→3) to a galactosyl residue that in turn is linked (1→3) to a glucosaminyl residue at the reducing end, which is also carrying a fucosyl residue at the 4-position.[CHEBI:62681]
term_xref : GTC:G00053MO|GlycoEpitope:EP0008|CID:53356732|CHEBI:62681|GlycoMotif:GGM.000024
synonyms : SLea|CA19-9|CA19-9 antigen|SLe^a|Slea
function : The carbohydrate determinants,Sialyl Lea and Sialyl Lex,which are frequently expressed on human cancer cells,serve as ligands for a cell adhesion molecule of the selectin family,E-selectin,which is expressed on vascular endothelial cells. These carbohydrate determinants are involved in the adhesion of cancer cells to vascular endothelium and thus contribute to hematogenous metastasis of cancer.[GlycoEpitope:EP0008]
disease_associations : pancreatic adenocarcinoma[GlycoEpitope:EP0008]|hepatocellular cancer[GlycoEpitope:EP0008]|cholangiocellular cancer[GlycoEpitope:EP0008]|gastric cancer|[GlycoEpitope:EP0008]colorectal cancer[GlycoEpitope:EP0008]|ovarian cancer[GlycoEpitope:EP0008]|lung cancer[GlycoEpitope:EP0008]|breast cancer[GlycoEpitope:EP0008]|uterine cancer[GlycoEpitope:EP0008]
wikipedia :
essentials_of_glycobiology :
Sialyl Lewis x
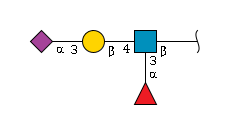
term (main_entry) : Sialyl Lewis x
glycan_dictionary_accession : GSD000151
glytoucan_accession : G00054MO
term_in_sentence : As sialyl Lewis X is a lignad of the selectin family, it has been proposed that sialyl Lewis X-rich colon cancer cells metastasize to the liver by adhesion to selectins on hepatic endothelial cells.[PMID:12828079]
publication : 12828079|8940172|10075923|19054801|9642161|31054033|15229399|29061785|16033070|10728707|9556613|8940172|9298692|18607721|22593503|25944902| 9642161|15780091|20401888
definition : A sialylated version of Lewis X antigen expressed on cell surfaces with structure alpha-Neup5Ac-(2->3)-beta-D-Galp-(1->4)-[alpha-L-Fucp-(1->3)]-beta-D-GlcpNAcis α-Neup5Ac-(2→3)-β-D-Galp-(1→4)-[α-L-Fucp-(1→3)]-D-GlcpNAc in which the anomeric configuration of the reducing-end N-acetyl-beta-D-glucosamine residue is beta. It is a ligand for selectins. [MESH:2030970],CHEI:71622
term_xref : GTC:G00054MO |GlycoEpitope:EP0012|CID: 44456859|CHEBI:71622|MESH:2030970|GlycoMotif:GGM.000022
synonyms : SLe(x)|SLe^x|SLex|Slex
function : Sialyl Lex was shown to be a specific ligand on human leukocytes for vascular E- and P-selectins,and was shown to mediate leukocyte recruitment.[GlycoEpitope:EP0012]|The determinant is also frequently expressed on human cancer cells,and is involved in the adhesion of cancer cells to vascular endothelium and thus contribute to hematogenous metastasis of cancer.[GlycoEpitope:EP0012]
disease_associations : colon cancer[GlycoEpitope:EP0012]|colorectal cancer[GlycoEpitope:EP0012]
wikipedia :
essentials_of_glycobiology :
Sialyl Lewis x-i
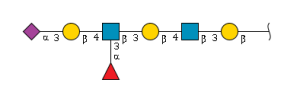
term (main_entry) : Sialyl Lewis x-i
glycan_dictionary_accession : GSD000152
glytoucan_accession : G06597DS
term_in_sentence : Univariate analysis showed that survival of NSCLC patients with high (more than 100 units/ml) sialyl Lewis X-i antigen levels was significantly poorer than that of patients with low antigen levels (P = 0.0001).[PMID:9815711]
publication : 9815711|9730796|11867833|9713476|2819663|9372327|9615762|1352835|2575680|9330526|8753037|2210805|12680155|11974864|10453898|2565704|17144590
definition :
term_xref : GTC:G06597DS|GlycoEpitope:EP0043|CID: 91855346|CHEBI:147019
synonyms :
function : hematogenous metastasis[GlycoEpitope:EP0043]|the progression of inflammation[GlycoEpitope:EP0043]
disease_associations : lung cancer[GlycoEpitope:EP0043]|ovarian cancer[GlycoEpitope:EP0043]|pancreatic adenocarcinoma[GlycoEpitope:EP0043]
wikipedia :
essentials_of_glycobiology :
Sialyl T antigen
term (main_entry) : Sialyl T antigen
glycan_dictionary_accession : GSD000153
glytoucan_accession : G65562ZE
term_in_sentence : The expression and functional analysis of the sialyl-T antigen in prostate cancer.[PMID:32583304]
publication : 32583304|31897478|31719620| 10365852|25727149|11523928|20407596|25464017|30864133|26391208|19811634|29796594|23725156|8905873|11192238|18275089|27037304|11949946|15604091|24118321
definition : Alpha-Neup5Ac-(2→3)-beta-D-Galp-(1→3)-alpha-D-GalpNAc is alpha-Neup5Ac-(2→3)-beta-D-Galp-(1→3)-D-GalpNAc in which the configuration at the GalNAc anomeric carbon is alpha. It has a role as an epitope.[CHEBI:65257]
term_xref : GlycoMotif:GGM.000013|GTC:G65562ZE|CID:44611726|CHEBI:65257
synonyms :
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
Sialylated biantennary
term (main_entry) : Sialylated biantennary
glycan_dictionary_accession : GSD000154
glytoucan_accession :
term_in_sentence : Simbucus nigra agglutinin or an antibody specific to sialylated lactosamine with a preference for Neu5Acalpha2-6Gal rather than Neu5Acalpha2-3Gal reduced sperm binding to oviduct isthmic cells, as did occupying putative receptors on sperm with sialylated biantennary glycans.[PMID:23115267]
publication : 23115267|31510952|29265394|31589880|3979568|26045275|29029079|30252064
definition : A binantennary glycan with one or two sialic acid residues as non-reducing terminal residues on one or both antennae. [RN]
term_xref :
synonyms : sialyl biantennary
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
Sialylated complex-type
term (main_entry) : Sialylated complex-type
glycan_dictionary_accession : GSD000155
glytoucan_accession :
term_in_sentence : By using 1H-NMR spectroscopy we were able to establish that our product contains a single N-linked biantennary , fully sialylated complex-type oligosaccharide , typical of human hepatomas.[PMID:1694115]
publication : 1694115|15201278|30471292|30969765|11058758|28342150
definition : Complex N-linked glycan that have added GlcNAc residues at both the α-3 and α-6 mannose sites and do not contain mannose residues apart from the core structure but with sialic acid residues.
term_xref :
synonyms : sialo-complex type
function : enhance the signaling activity of soluble intercellular adhesion molecule-1 in mouse astrocytes [PMID: 15201278] Appears in IgG-Fc.[PMID:20647032 ]
disease_associations :
wikipedia :
essentials_of_glycobiology : Chapter 10
Sialylated LacdiNAc
term (main_entry) : Sialylated LacdiNAc
glycan_dictionary_accession : GSD000192
glytoucan_accession : G55583HW
term_in_sentence : The major non-reducing epitopes in the complex-type glycans are: Gal beta 1-4GlcNAc (lacNAc), GalNAc beta 1-4GlcNAc (lacdiNAc), NeuAc alpha 2-6Gal beta 1-4GlcNAc (sialylated lacNAc), NeuAc alpha 2-6Gal beta 1-4GlcNAc (sialylated lacdiNAc), Gal beta 1-4(Fuc alpha 1-3)GlcNAc (Lewisx), and GalNAc beta 1-4(Fuc alpha 1-3)GlcNAc (lacdiNAc analogue of Lewisx).[PMID:7592613]
publication : 7592613|34192302|19756298|16286643
definition :
term_xref : GlycoMotif:GGM.000050
synonyms : sialylated-LacdiNAc|Sialylated LDN
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
Sialylated tetraantennary
term (main_entry) : Sialylated tetraantennary
glycan_dictionary_accession : GSD000156
glytoucan_accession :
term_in_sentence : A small amount of sialylated tetraantennary oligosaccharide was detected.[PMID:16321355]
publication : 16321355|1940447|27501865
definition : A tetraantennary glycan with four GlcNAc branches linked to the core and the addition of one sialic acid residues.
term_xref :
synonyms :
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
Sialyl-Tn antigen

term (main_entry) : Sialyl-Tn antigen
glycan_dictionary_accession : GSD000157
glytoucan_accession : G36123IU
term_in_sentence : The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-β secretion from monocytes/macrophages through the DAP12-Syk pathway.[PMID:23035012]
publication : 23035012|29273237|15770530|31595388|26617889|1720922|15466199|10971172|14652017|8616877|1543361|16965854|21566901|9269833|9834267|24970145|22367369|10778170|30018351|28628803|16149605|32134186|28632300|11308020| 10365852|17195456|23567325|11014575|2342728|8575847|1851822|11735169|20960853|7846011
definition : N-acetyl-alpha-neuraminyl-(2→6)-N-acetyl-alpha-D-galactosamine is an N-acetyl-alpha-neuraminyl-(2→6)-N-acetyl-D-galactosamine in which the carbon bearing the anomeric hydroxy group has alpha configuration. It has a role as an epitope. It derives from a N-acetyl-alpha-D-galactosamine and a N-acetyl-alpha-neuraminic acid.[CHEBI:61818]
term_xref : GTC:G36123IU|GlycoEpitope:EP0022|CID:51351800|CHEBI:61818
synonyms :
function : apoptosis of monocyte-derived dendritic cells[GlycoEpitope:EP0022]|decrease of adhesion and increase of migration[GlycoEpitope:EP0022]|increase of tumor growth[GlycoEpitope:EP0022]|associated with morphological changes, decreased growth and increased migration of MDA-MB-231 cells.[GlycoEpitope:EP0022]
disease_associations : anaplastic meningioma[GlycoEpitope:EP0022]|Barrett's esophagus[GlycoEpitope:EP0022]|bladder cancer[GlycoEpitope:EP0022]|breast cancer[GlycoEpitope:EP0022]|colon cancer[GlycoEpitope:EP0022]|colorectal cancer[GlycoEpitope:EP0022]|erythroblastic leukemia[GlycoEpitope:EP0022]|esophageal cancer[GlycoEpitope:EP0022]|gastric cancer[GlycoEpitope:EP0022]|helicobacter pylori chronic gastritis(HpCG)[GlycoEpitope:EP0022]|liver cancer[GlycoEpitope:EP0022]|iterome cancer[GlycoEpitope:EP0022]|ovary tumor[GlycoEpitope:EP0022]|pancreas cancer[GlycoEpitope:EP0022]
wikipedia :
essentials_of_glycobiology : Chapter 56
SLex Core 2 O-glycan
term (main_entry) : SLex Core 2 O-glycan
glycan_dictionary_accession : GSD000158
glytoucan_accession : G97345NY
term_in_sentence : In particular, the sLeX-modified core 2 O-glycan structure C2-O-sLeX has been directly demonstrated to confer significantly higher affinity selectin binding than sLeX.[PMID:15596301]
publication : 15596301
definition :
term_xref : GlycoMotif:GGM.000053|GTC:G97345NY
synonyms : SLe^x Core 2 O-glycan
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
SSEA-1
term (main_entry) : SSEA-1
glycan_dictionary_accession : GSD000159
glytoucan_accession : G85497PI
term_in_sentence : Our findings suggest that expression of SSEA-1 immunoreactivity in thyroid neoplasms was associated with more aggressive thyroid carcinomas.[PMID:27550342]
publication : 27550342|25564944|30987116|10778174|19427293|24923741|28092949|16499901|10470652|18787210|2885312|16149609|17003364|27363517|15505339|27990704|2568298|20491542|23572256|1977666|9553168|20458727|2566639|14577357|20960856|15547746|9096265|30639212|1357195|1359630|20181261|20508065|2907526|22624699|22988836|17183690|27188587|7908065|10400391|6137429|1979260|2898921|14736812|30919971
definition :
term_xref : GTC:G85497PI |GlycoEpitope:EP0042|CID:91851144|CHEBI:154343
synonyms :
function : This determinant is specifically recognized by the antibody raised against stage-specific embryonic antigen-1 (SSEA-1)[GlycoEpitope:EP0042]. The SSEA-1 antibody requires Lex hapten carried by i-antigenic structure (e.g.,nor-nLc6Cer).[GlycoEpitope:EP0042]
disease_associations :
wikipedia :
essentials_of_glycobiology : Chapter 10
SSEA-3
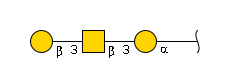
term (main_entry) : SSEA-3
glycan_dictionary_accession : GSD000160
glytoucan_accession : G25129BT
term_in_sentence : Glycosphingolipids (GSLs), such as the globo-series GSLs stage-specific embryonic antigen 3 (SSEA-3), SSEA-4, and Globo-H, are specifically expressed on pluripotent stem cells and cancer cells, and are known to be associated with various biological processes such as cell recognition, cell adhesion, and signal transduction.[PMID: 31817926]
publication : 31817926|30271856|32684574|26677875|23175153|32525407| 29227830|[https://pubmed.ncbi.nlm.nih.gov/18773292 18773292 18773292]|25561682|10837462|2412924|[https://pubmed.ncbi.nlm.nih.gov/21149348 21149348 21149348]|[https://pubmed.ncbi.nlm.nih.gov/17008424 17008424 17008424]|[https://pubmed.ncbi.nlm.nih.gov/2874662 2874662 2874662]|25171543|31304790|31642560|30484221|30525067|21161592|27325407|28170296|29637816| 27318475|31346146|7648586|24236059|21964054|27456773|30558911|21559451|29312455|27549315|30599359|6141938|28883415|30733757|26041528|16643871
definition : An amino trisaccharide consisting of β-D-galactopyranose, 2-acetamido-2-deoxy-β-D-galactopyranose and α-D-galactopyranose residues joined in sequence by (1→3) glycosidic linkages.[CHEBI:149071]
term_xref : GTC:G25129BT|GlycoEpitope:EP0039|CID:91855781|CHEBI:149071|GlycoMotif:GGM.000079
synonyms :
function :
disease_associations : embryonal carcinoma[GlycoEpitope:EP0039]|teratocarcinoma[GlycoEpitope:EP0039]|yolk sac carcinoma[GlycoEpitope:EP0039]
wikipedia :
essentials_of_glycobiology :
SSEA-4
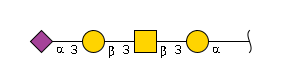
term (main_entry) : SSEA-4
glycan_dictionary_accession : GSD000161
glytoucan_accession : G84263XI
term_in_sentence : Glycosphingolipids (GSLs), such as the globo-series GSLs stage-specific embryonic antigen 3 (SSEA-3), SSEA-4, and Globo-H, are specifically expressed on pluripotent stem cells and cancer cells, and are known to be associated with various biological processes such as cell recognition, cell adhesion, and signal transduction.[PMID:31817926]
publication : 27845370|31817926|26295543| 25171543|25978997|30296654|25853231|24862940|31243630|28736232|22895512|24578244|29478902|23574147|23768762|27797400|29227830| 18773292|25123923|22924692|22665977|23330736|31831622|31916611| 2412924|16033656|24866102| 20458727|[https://pubmed.ncbi.nlm.nih.gov/21149348 21149348 21149348]|26940129| 17008424| 2874662|23301556|27836003|19118716|24550271|32988880|22743677|20683402|17062733|23570331|30310530|31545316|23509763|24364909|18273440|28985528|22266579
definition :
term_xref : GTC:G84263XI|GlycoEpitope:EP0044|CID: 91845478|CHEBI:154843
synonyms :
function :
disease_associations : teratocarcinoma[GlycoEpitope:EP0044]
wikipedia :
essentials_of_glycobiology : Chapter 47
Sulfated LacdiNAc
term (main_entry) : Sulfated LacdiNAc
glycan_dictionary_accession : GSD000191
glytoucan_accession : G18464VS
term_in_sentence : The mRNA expression of the enzymes related to the formation of sulfated LacdiNAc:β(1,4)-N-acetylgalactosaminyltransferases 3 (B4GALNT3) and 4 (B4GALNT4), carbohydrate sulfotransferases 8 (CHST8) and 9 (CHST9); and β(1,4)-galactosyltransferase 1 (B4GALT1) in these cells was confirmed by RT-PCR (Fig. S4).[PMID:31558607]
publication : 31558607|27318476|19756298|11279168|26688390
definition :
term_xref : GlycoMotif:GGM.000049
synonyms : 4'-O-sulfated LacdiNAc|4'-sulfated LDN|sulfated LDN
function :
disease_associations :
wikipedia :
essentials_of_glycobiology :
Sulfatide
term (main_entry) : Sulfatide
glycan_dictionary_accession : GSD000162
glytoucan_accession : G72548RZ
term_in_sentence : In a previous study we found that sulfatide was efficient in lowering HIV-1 viral loads in SCID mice engrafted with human fetal liver/thymus tissues (SCID-hu).[PMID:23550344]
publication : 23550344|18465098|26542149|15158666|10434709|22619219|29676479|30196830|16143863|1052451|30400820|29887538|26981544|17986152|31739035|19941861|24578319|30531843|25645724|31197846|22821828|27861899|31037952|23593400|23604989|17855742|25966258|25989600|9657940|29497057|12838515|25170267|9878314|20554435|1410493|26585924|17257892|17044925|21089387|11445552|22977233|21437360|17179664|6273571|8370675
definition :
term_xref : GlycoMotif:GGM.000063|GTC:G72548RZ
synonyms :
function :
disease_associations :
wikipedia :
essentials_of_glycobiology : Chapter 47